True navigation is defined as the
ability of an animal to return to its original location after
displacement to a site in unfamiliar territory, without access to
familiar landmarks, goal emanating cues, or information about the
displacement route. This ability, which has been demonstrated in
vertebrates (Rodda & Phillips 1992) and one invertebrate (spiny
lobster; Boles & Lohmann, 2003), requires both a sense of direction
(“compass”), and a sense of geographic position (“map”).
Vertebrates
have multiple compass systems (sun, star, polarized light and magnetic
compasses; Wiltschko & Wiltschko, 1972; Emlen, 1975;
Schmidt-Koenig, 1979; Able, 1980; Phillips, 1986b; Moore, 1987;
Wiltschko & Wiltschko 1995a; Lohmann & Lohmann, 1996).
Factors that determine which of these compass systems is used at any
given time include weather conditions, time of day, and past
experience. Each of these compass systems requires different
sensory detection/processing mechanisms, e.g., a time compensation
mechanism for the sun compass (Saint Paul, 1953; Phillips &
Waldvogel, 1988s; Schmidt-Koenig, 1990), an ability to relate the
alignments of star patterns to the axis of celestial rotation for the
star compass (Sauer & Sauer, 1960; Emlen, 1970; Wiltschko, et. al,
1987; Weindler et. al, 1996u), and specialized sensory receptors
capable of detecting the plane of polarized light and alignment of the
geomagnetic field for the polarized light and magnetic compasses
(Brines & Gould 1982; Phillips & Moore, 1992q; Able & Able,
1993). Each compass system also incorporates to varying degrees
both innate and learned components (Gwinner & Wiltschko, 1978;
Helbig, 1992; Bletz et. al, 1996; Helbig, 1996; Able & Able,
1997). To avoid systematic errors in the direction of orientation
when switching between compasses, each of these systems must be
calibrated with respect to a common reference system. In birds,
where the integration of compass information is best understood, the
primary compass calibration reference appears to be derived from
celestial cues, probably polarized patterns present at sunset and,
possibly also sunrise (Muheim et. al, submitted).
Accurate
navigation only requires that the map and compass are in register
with one another, i.e., that the animal navigator is able to
associate a geographic position specified by the map with a compass
bearing that will enable it to return to the origin of a
displacement or to some other predetermined destination. As noted
above, however, birds appear to use a global reference system
derived from celestial polarized light patterns to calibrate their
compass and presumably, therefore, also their map systems.
The key feature
of a navigational map is that it can be extrapolated to unfamiliar
sites and used to orient homeward when there is no contact with
familiar “landmarks” or reference points, or with goal-emanating
cues. This definition of a navigational map applies to both
olfactory and magnetic gradient maps (see below), but not olfactory
mosaic maps (Griffin, 1952; Gould, 1982; Wehner, 1998; Able, 2000) or
other forms of “piloting” or “place navigation” involving the use of
familiar visual, olfactory or magnetic reference points. A
“gradient” map requires an animal to learn the alignment and,
possibly, steepness of one or more environmental gradients within
its home range or territory, and to extrapolate these gradient(s)
beyond its area of familiarity. Comparison of the value of such a
map component at an unfamiliar site with that of the “home value”
provides information about the animal’s position along the gradient
in relationship to home. Non-parallel gradients of two or more
different map components would enable an animal to determine its
position in two dimensions (bicoordinate position fixing).
The home value of
each map component provides the reference necessary to determine the
direction (and distance) of displacement along each extrapolated
gradient. Since both magnetic and olfactory gradients change over
time (Skiles, 1985; Courtillot et. al, 1997; Waldvogel, 1987; Ganzhorn,
1995; and see below), the home value(s) of an olfactory or magnetic
map have to be updated periodically. The animal, therefore,
must have a means of distinguishing home from non-home locations
(“home recognition” cues) that are independent of the cues used to
derive geographic position (“map” cues). Manipulation of home
recognition cues may produce dramatic changes in homing orientation
and/or homing success that can easily be mistaken for an effect on
cues underlying the map (see below).
A unified theory
of spatial navigation requires integration of mechanisms used to
guide movements within familiar configurations of landmarks
(piloting, place maps, bearing maps, olfactory mosaic maps) with
those used for homing from unfamiliar sites (bicoordinate
navigation, gradient maps). However, attempts to develop a
unified theory have generally failed to address the differences in
the processing of sensory information required for these two types
of response. For piloting or place navigation, spatial
position in derived with respect to a configuration of familiar
landmarks or reference points, with the critical values being the
directions and, in some cases, distances to those reference points
(Figure 1A), or related features such as the size and appearance of
a visual landmark. A variant of this type of map, referred to
as a “bearing map” (Jacobs & Schenk,
2003), defines locations in terms of their compass bearing
(distance?) from familiar reference points (Figure 1B). In contrast,
for navigation using a gradient map, spatial position is derived
from the difference in the local value of each map component
relative to the home value of that component (Figure 1C and D).
 |
|
Figure 1. Maps of spatial
position. A) Place map, B) Bearing map, C) Bicoordinate
gradient map, D) Simple gradient map. ‘an’
indicates a measure of relative spatial relationship to the
organism at a given site (in A) or a compass bearing
(distance?) from a familiar reference site (in B). “x” and
“y” are the values of two non-orthogonal environmental
gradients. |
Because large scale
gradients of magnetic and olfactory cues are too weak for an animal
to detect directly, the alignment (and possibly steepness) of such
gradients would have to be learned by obtaining multiple “point
samples” at different sites that are in a known spatial relationship
to one another (see Phillips & Deutschlander, 1987; Phillips, 1996). For
such large scale gradients of magnetic (Phillips, 1996) or olfactory
(Wallraff, 2004) cues, the origin or source of the gradient, and
thus the direction and distance from that source, is undefined in
the two dimensional world of the navigating animal.
Some types of cues may function in
both local place maps and large-scale gradient maps. For
example, local odor sources could be used in a “mosaic” olfactory
map (comparable to a place map; Walraff, 2003, see
also Bingman et. al, this volume). However, this type of map is
limited in spatial scale by the detection range of discrete odor
sources, and the spatial heterogeneity of odor distributions
(Waldvogel, 1987; Ganzhorn, 1995). Over a larger spatial scale,
other types of olfactory information (e.g., the ratio of odors from
extended sources; Walraff, 2004) have been proposed to function in a
gradient map that can be extrapolated beyond an individual’s area of
familiarity.
Spatial
variation in the magnetic field has also been suggested to provide map
information over different spatial scales (Phillips, 1996). Over
distances > 50-75 km (depending on the locality), regional
gradients in magnetic field components such as inclination, total
intensity and, possibly, declination could be used to provide unicoordinate or bicoordinate map information. At the other
extreme, local gradients extending over distance of < 5-10 km, which
often differ from the regional gradient in both direction and
steepness, may provide the basis for a small scale gradient map.
Recognizable local “anomalies” in the magnetic field may also provide
reference points (possibly in combination with visual or olfactory
cues) for a place or bearing map (Ganzhorn, 1990; Walker et. al,
2002). At intermediate distances (from 10-20 km to 50-70 km),
local and regional variation tend to be similar in magnitude, so both
higher and lower magnetic field values are likely to be encountered in
all directions. At these distances, the magnetic field is
unlikely to provide a reliable source of map information (Phillips,
1996).
 |
|
Figure 2.
Effects of distance on the accuracy of pigeon homing (data
from Schmidt-Koenig, 1970). Solid black symbols show
homeward component calculated from the pooled vanishing
bearings of birds released at four sites around a loft in
Durham, NC USA (solid black line) and a loft in Frankfurt,
Germany (dashed black line). Horizontal dotted arrows show
predicted range of magnetic gradient maps (red), olfactory
gradient maps (green), and familiar landmarks (purple), and
range of distances at which pulse remagnetization was found
to affect pigeon homing (blue). Vertical dashed lines show
boundaries of predicted ranges of magnetic gradient maps
(red) and of range at which effects of pulse remagnetization
were observed (blue). Question marks indicate where
boundaries are not specified (green) or dependent on
experience (purple), or data are not available (blue). |
Given the spatial
heterogeneity of potential map cues, animals that differ in mode of
locomotion and range of movement are likely to use different types
of maps to determine spatial position. Moreover, the same
species may rely on different sources of map information for short
and long distance movements. Interestingly,
Schmidt-Koenig (1966; 1970) found that homing pigeons at a loft in
the United States and one in Germany showed markedly poorer homing
orientation from sites at intermediate distances from the loft
(30-70 km) in comparison to sites both closer and further away
(Figure 2), suggesting that different (and, at these sites,
non-overlapping) navigational mechanisms may be used for short and
long-distance homing (see also Matthews, 1963). Gradient maps may also differ in
complexity (and, consequently, in underlying neural architecture) in
different animals. At one extreme, a bicoordinate map may provide
estimates of actual position that vary continuously in both
direction and distance from the animal’s “home” or final destination
(bicoordinate position fixing; Figure 1C). At the other extreme, a
relatively simple stimulus/response system that triggers a limited
number of discrete behaviors (“orient to the southwest if the
values of both map coordinates are greater than the home values";
i.e., X+Y+ in Figure 1D) may suffice to produce
accurate homing under some conditions (K. Lohmann, personal communication).
Interestingly, a simple gradient map like that shown in Figure 1D may
be functionally indistinguishable from a course-grained bearing map
in which compass bearings are associated with large areas or sectors
around an individual’s home.
Finally, sites that an animal
initially localizes by means of one type of map (e.g., a magnetic or
olfactory gradient map; Figure 1C) may subsequently be incorporated
into a grid of familiar reference points (i.e., place map; Figure 1A),
and/or assigned a particular directional relationship to home or
other final destination (bearing map; dashed arrow in Figure 1B)
(see Jacobs and Schenk 2003). Subsequent recognition of such sites
may involve a unique “signature” of cues from the same, or a
different, sensory modality, including local olfactory, visual, or
magnetic cues (Ganzhorn, 1990; Burt et. al, 1997; Walker et. al,
2002). Like home/non-home recognition cues, the role of cues in
providing unique labels or signatures of familiar sites (“site
labeling cues”) may be difficult to distinguish from that of cues
used to initially establish the spatial position of that site (“map
cues”).
Both olfactory
and magnetic cues have been proposed as potential sources of
gradient map information (Guilford et. al, 1998; Hays et. al, 2003).
Olfactory effects on homing have been relatively easy to document,
although the question of whether olfactory cues are involved in the
map is more controversial. Magnetic field effects on
homing have been more difficult to demonstrate reliably. However,
there is a growing body of evidence for the magnetic field’s
involvement in the map component of homing, due largely to the
development of experimental systems that have made it possible to
study map-based homing (true navigation) under controlled laboratory
conditions (Fischer et. al, 2001; 2003; Phillips et. al, 2002b; Boles
et. al, 2002; Lohmann et. al, 2004).
More generally,
attempts to understand the sensory basis of navigational gradient
map(s) have been hampered by an overly simplistic view of animal
navigation which has largely ignored components of navigation systems
other than the map and compass. As a consequence, investigators
often fail to consider alternative hypotheses that involve (e.g.)
effects on cues used for home vs. non-home recognition, and for
recognition or “labeling” of familiar sites. By discussing such
alternatives, we hope to stimulate future studies that will clarify
the complex interactions of different types of sensory information
that underlie animal navigation and, specifically, the navigational
map or maps.
|
|
 |
|
|
Figure 3. Effects of
exposure to bottled air and “nonsense odors” during displacement
to unfamiliar release sites on the homeward orientation of
pigeons. Vanishing bearings from four release sites
plotted as deviations from the mean vector bearing of controls
at each site (J.B. Phillips, J. Ganzhorn & K Schmidt-Koenig,
unpublished data) |
A variety of
vertebrates have been shown to recognize and orient towards odors
associated with discrete food sources, nesting sites, or breeding
areas over distances from a few hundred meters to a kilometer or
more (e.g., Clark & Shaw, 1992;
Joly & Miaud, 1989;
1993; Nevitt et. al, 2004).
In birds, discrete odor sources have also been hypothesized to play a
role in providing map information over longer distances, forming a
“mosaic” map of olfactory space. In a mosaic olfactory map,
discrete odor sources function in the same manner as the reference
sites (landmarks) in a place map. Like distant visual landmarks,
it has been hypothesized that the location of discrete odor sources can
be determined without the individual actually visiting the site where
the odors originate, by associating particular odors or combinations of
odors with winds blowing from different directions (but see
below). Even in birds, however, the range over which a mosaic
olfactory map can operate is likely to be limited to at most a few tens
of kilometers (Waldvogel, 1987; Ganzhorn, 1995; Bingman, this
volume). For animals confined to the substrate, olfactory cues
are likely to function over considerably shorter distances, perhaps
only a few hundred meters (Joly & Miaud, 1989; 1993). This is
especially true in forested habitats where turbulence in the understory
eliminates any consistent relationship between overhead wind direction
and the direction of air currents at ground level
(e.g., Hutchison & Hicks 1985;
Baldocchi, 1989).
At the other extreme, Walraff
(2004) proposed that birds may use an olfactory gradient map based
on the spatially varying ratio of certain atmospheric chemicals that
can extrapolated to unfamiliar areas over distances of hundreds or
even thousands of kilometers.
A variety of
techniques have been used to investigate whether olfactory cues are
involved in the map component of navigation, including: (1)
olfactory deprivation (anesthesia or chemical ablation of the
olfactory mucosa, sectioning of the olfactory nerve, exposure to
filtered or bottled air during displacement to a release site, and
masking of natural odors with strong odorants), and (2) altering the
relationship between wind direction and natural or artificial odors
to which birds are exposed in their home loft, in some cases with
exposure to the same or different odors at an unfamiliar release
site prior to release.
Olfactory
deprivation either during displacement to a release site and/or
during the homeward flight has been shown to cause increased
scatter in initial homing orientation and/or decreased homing
success in a large number of experiments (e.g., Sinsch, 1990a; 1990b; Grant et. al, 1968; Wallraff, 1990;
2004; Wallraff et. al, 1995). However, these effects are not
universal (e.g., Sinsch, 1990b; Ganzhorn, 1990; 1992; Wiltschhko et.
al, 1989). For example, in homing pigeons the effects of olfactory
deprivation have been shown to vary with loft location and/or early
experience, and with characteristics of individual release sites (Ganzhorn,
1990; 1992; Wiltschko et. al, 1989). More importantly, experiments
showing effects of olfactory deprivation on homing have often failed
to rule out alternative explanations involving effects on sensory
systems other than olfaction (Mora et. al, 2004), and/or olfactory
effects on component(s) of the bird’s navigational system other than
the map (e.g., Wiltschko, 1996; Phillip & Waldvogel, 1988; Ganzhorn;
1990; and see below).
Two
alternatives explanations for effects of olfactory deprivation on
homing warrant particular attention. In birds, a specialized
magnetoreception system associated with the trigeminal nerve has been
implicated in the map component of homing (e.g., Beason and Semm, 1987;
1996; Munro et. al, 1997a; 1997b; Beason et. al, 1997; Walker et. al,
1997; and see below). In conditioning experiments with pigeons,
Mora et al. (2004) found that anesthesia of the olfactory mucosa, a
technique used to produce olfactory deprivation in behavioral studies
of pigeon homing (see Walraff, 2004), also blocks trigeminal nerve
mediated responses to magnetic stimuli. Consequently, studies of
homing orientation using this technique may inadvertently deprive birds
of magnetic, as well as olfactory, information. Secondly,
deprivation of olfactory information may deprive birds of the ability
to distinguish home from non-home, or to recognize the olfactory
signature of familiar sites (see earlier discussion). The
possibility of such effects is suggested by preliminary experiments
with homing pigeons (Phillips, Ganzhorn, & Schmidt-Koenig,
unpublished data). Previous experiments have shown that
preventing access to olfactory cues during displacement to an
unfamiliar release site can, under some conditions, affect the homing
orientation of pigeons (e.g., Wiltschko et. al, 1989; Papi, 1990;
Walraff, 2004). In an attempt to distinguish map-related and
non-map-related effects of olfactory deprivation, experienced homing
pigeons were subjected to one of three treatments during displacement
to four release sites arrayed symmetrically around the home loft.
Controls were exposed to natural odors during displacement. One
experimental group was exposed to synthetic bottled air during
transport that eliminated access to natural odors (“bottled air”
group). A second experimental group was also exposed to synthetic
bottled air during displacement, but a series of artificial odors
(peppermint, spearmint, orange, clove, etc.) was introduced into the
air stream (“nonsense odor” group). The odor was changed every 5
minutes during the displacement to prevent habituation. The same
sequence of artificial odors was presented to the birds during
transport to each of the release sites, so that even if one of the
nonsense odors occurred naturally in a particular direction from the
home loft, exposure to this odor would not produce consistent homeward
orientation when departure directions were pooled from all four release
sites.
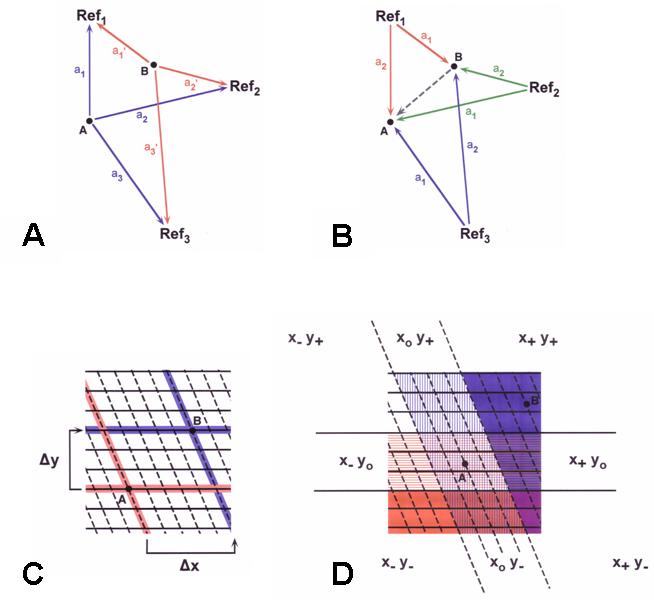
Figure
3A & B shows the vanishing bearings (“initial orientation”) of the
two experimental treatments plotted at deviations from the mean
vector bearing of controls tested at the same release site. Plotting the
bearings in this way eliminates any effect of site-specific
deviations from the home direction. The distribution of vanishing
bearing from bottled air birds differed significantly from that of
controls (Figure 3A). In contrast, the distribution of
vanishing bearings of nonsense odor
birds was indistinguishable from that controls (Figure 3b). While by
no means conclusive,
these findings illustrate the importance of carrying out experiments
to determine whether exposure to unfamiliar odors that provide no
information about the position of the release site relative to the
home loft can mimic the effect of exposure to natural odors.
If so, this would suggest that olfactory cues play a role in a
component(s) of the animal’s navigation system other than the map.
For example, exposure to non-home odors could activate the birds’
navigational system, causing the birds to access stored map and
compass information and/or to attend to non-olfactory
information necessary for homing. The crucial point here is that
despite the large number of experiments showing effects of olfactory
deprivation on homing (experiments involving treatments such as
sectioning of the olfactory nerve, occlusion of the nostrils,
anesthetization of the olfactory mucosa, ablation of the olfactory
mucosa with zinc sulfate, and exposing birds to filtered or bottled
air during displacement; for reviews see Papi, 1990; Walraff, 2004),
critical controls have not been carried out to determine whether
these effects are due to the involvement of olfactory cues in the
map.
The same criticism can be leveled at experiments comparing the
orientation of groups of pigeons exposed to natural odors at a
“false” release site and then rendered anosmic before being transported to a site in the opposite
direction from the home loft for testing (e.g., Benvenuti & Walraff,
1985; Kiepenheuer, 1985).
|
|
 |
|
|
Figure 4: Initial orientation of homing
pigeons after housing in deflector and control lofts (data
from Waldvogel & Phillips 1982).A) Initial orientation of
pigeons housed in deflector loft that produced a clockwise (CW)
rotation of wind direction and reflected light cues (see
inset). B) Initial orientation of pigeons housed in control
loft without deflector panels. C) Initial orientation of
pigeons housed in deflector loft that produced a
counterclockwise (CCW) rotation of wind direction and
reflected light cues (inset). |
In addition to
olfactory deprivation, a number of experiments with homing pigeons
using techniques that alter the relationship between wind direction
and either natural or artificial odors have produced findings
consistent with the olfactory map hypothesis. Like the olfactory
deprivation experiments, however, most, if not all, of these
experimental findings are open to alternative interpretations. Here
we focus on one type of such experiments, the so-called
“deflector loft” experiments. In these experiments, three lofts
were constructed with sides of wire mesh and vertical louvers that
allowed air to pass through unimpeded. Two of the lofts were
equipped with large “deflector panels” attached to the sides that
rotated incoming winds in either a clockwise (CW) or
counterclockwise (CCW) direction (Figure 4). We focus on the
deflector loft experiments, because: (1) birds are exposed to
natural, rather than artificial, odors, (2) shifts in wind direction
are produced passively, thus avoiding potential artifacts associated
with fans (noise, infrasound, turbulence, electromagnetic fields, etc), (3) the
predicted effects of the deflector lofts are unambiguous, i.e., if
pigeons learn the distribution of odor sources by associating the
presence of specific odors with winds arriving from particular
directions, then rotation of wind direction in the deflector lofts
must produce a corresponding shift in the birds’ olfactory
map and, therefore, in the direction of homing orientation (Phillips
& Waldvogel, 1982), and (4) the deflector loft effect has been
replicated by laboratories in Italy, German and the
United States (e.g., Baldaccini et. al, 1975; Ioalé et. al, 1978; Kiepenheuer,
1978; Waldvogel et. al, 1978).
Although the
deflector loft experiments are frequently cited as support for the
olfactory map hypothesis (Walraff, 2004), several findings call this
conclusion into question.
(1)
Kiepenheuer (1979)
prevented deflector loft birds
from detecting olfactory cues by anesthetizing
the olfactory mucosa or plugging the
nostrils. Despite their inability to detect natural odors, the
birds exhibited shifted orientation indistinguishable from that of
pigeons that had been housed in the deflector lofts but were able to detect
natural odors. These findings indicate that the deflection of
initial orientation of deflector loft birds is not a response to
olfactory cues.
(2)
Kiepenheuer (1982)
removed the vertical
louvers from the sides of the deflector lofts (leaving only wire
mesh and the deflector panels), resulting in a “whirlwind” pattern
of wind flow within the lofts that eliminated any consistent
relationship between prevailing wind direction and the wind
direction experienced by birds at different locations
within
the loft. Again the deflection of initial orientation was
unaffected, suggesting that associating odors with wind direction does not play a role in the deflector loft
effect.
(3)
Phillips & Waldvogel (1982)
showed that the deflected initial orientation of pigeons held in
deflector lofts for 5-7 days (“short-term” residents) was a response
to light cues reflected from the deflector panels, rather than
wind-born cues. The deflected orientation of “permanent residents”
birds was also shown to be a response to the altered patternd of light cues
visible to the birds in the deflector lofts, rather than
wind-borne odors (Phillips & Waldvogel 1991). Phillips & Waldvogel
(1982, 1988) presented evidence that the deflector loft effect
results from rotation of a polarized-light-based reference system
used to calibrate the sun compass. Subsequent experiments with
migratory birds have demonstrated the use of polarized light cues to
calibrate the sun compass (Moore & Phillips 1993).
(4)
Waldvogel
and Phillips (1982) found that the shift in the initial orientation of
permanent resident deflector loft birds was present when birds were
released under sunny skies, but not under overcast. Since pigeons
are unlikely to rely on different source(s) of map information under
sunny and overcast conditions, these findings provide further evidence
that the deflector loft effect is linked to the use of the sun compass,
rather than an olfactory map.
The results of
these four experiments demonstrate: a) that the deflector loft
effect is caused by rotation of light, rather than olfactory, cues, and
b) that wind-borne cues perceived in the home loft do not play a
role in the navigational system of pigeons, at least under the
conditions used in these experiments. Despite the consistency of
the findings outlined above, the results of a fifth type of
deflector loft experiment has been widely cited as evidence for an
olfactory map.
In pigeons,
olfactory input projects to the ipsalateral hemisphere and from
there, via
the anterior commissure, to the contralateral hemisphere. Foà et al.
(1986) sectioned the anterior commissure of homing pigeons,
restricting olfactory input from each nostril to one hemisphere.
After recovery, “split brain” birds were housed alternately in the
CW and CCW deflector lofts. While housed in the CW loft, the
birds’ right nostrils were plugged, so olfactory information reached
only the left hemispheres. While housed in the CCW loft, the birds’
left nostrils were plugged, so olfactory information reached only
the right hemispheres. When the birds were released at an
unfamiliar site with their right nostrils plugged, their initial
orientation was deflected CW. When the birds were
released with their left nostrils plugged, their initial orientation
was deflected CCW. These findings are consistent with
the birds having established independent CW- and CCW- rotated
olfactory maps on the two sides of the brain (Foà et. al, 1986), and
appear to contradict those of the four experiments described above.
Like the effects of olfactory
deprivation, however, there are a number of alternative explanations
for the results of the “split brain” experiments. Here we discuss
one such possibility. As discussed earlier, olfactory cues may play a role in
a component of the pigeon’s navigational system other than the map,
such as distinguishing home from non-home (Figure 3). We propose that
calibration of the pigeon’s sun compass (which is altered by
light cues in the deflector lofts; see earlier discussion), and
access to this stored calibration information, is triggered by
exposure to home and non-home odors (respectively). According to
this scenario, the effects of plugging the right or left nostril in
the split brain experiments are a consequence of storing and
accessing differently calibrated sun compass systems on the two
sides of the brain. In contrast to an effect on the olfactory map
(as
proposed by Foà et. al, 1986), this explanation reconciles the split
brain experiments with the earlier deflector loft results.
Evidence that the deflector loft
effect is not due to wind-borne odors appears to be contradicted by
other “wind redirection” experiments with homing pigeons (reviewed
by Papi, 1990; Walraff, 2004). However, here again, alternative
hypotheses involving both olfactory and non-olfactory cues have been
largely ignored. For example, the effects observed in these
experiments could be caused by: a) light cues
reflected from glass or Plexiglas panels (Phillips & Waldvogel,
1988), b) aversive stimuli associated with electric fans
(electromagnetic fields, air turbulence, infrasound, etc.), or c)
pigeons using distinctive odors to “label” wind direction.
In summary, the
literature does not provide compelling evidence for the involvement
of olfactory cues in a long-distance gradient map. In birds like
homing pigeons, there remains the possibility that olfactory cues
play a role in a short-distance “mosaic” map, comparable to a place
or bearing map (Ganzhorn, 1990; 1992). However, the findings of the
deflector loft experiments make it unlikely that either type of
olfactory map involves natural wind borne odors reaching pigeons in their
home loft (see earlier discussion). As in the examples discussed
earlier, other experiments that have provided evidence consistent with an
olfactory mosaic map require additional controls to rule out
alternative explanations involving effects on both olfactory and
non-olfactory cues. Particular attention should be paid to the
possibility that specific odors encountered during displacement to
or upon arrival at a release site may provide one type of “site
labeling” cue that helps identify previously visited localities or
regions (Ganzhorn, 1990) and/or play a role in activating the
animal's navigations systems (Figure 3), rather that providing remote (i.e.,
wind-borne) information about the location of distant odor sources
(see earlier discussion). Clearly, a better understanding of the
role of olfactory cues in both map and non-map components of animal
navigation systems is crucial to interpreting the effects of a
variety of experimental treatments, including ablations of brain
regions involved in processing both spatial and olfactory
information (e.g., Bingman et. al, 1996; Bagliardo et. al, 1999; 1997;
2004; Ioale et. al, 2000).
On a global scale, magnetic
field intensity varies from a minimum of 20,000-25,000 nT at the magnetic equator to a maximum of
60,000-65,000 nT at the poles, and magnetic inclination varies from
0o (horizontal) at the equator to 90 o
(vertical) at the poles. Yeagley (1947; 1951) was the first
to suggest that spatial variation in the magnetic field could
provide homing pigeons with a source of north-south position (see also Moore, 1980;
Gould, 1985; Walcott, 1991). There are, however, a number of
potential problems with the use of a magnetic map, especially for
organisms that must resolve differences in spatial position on the
order of a kilometer or less (Phillips, 1996)
(1) Magnetic field gradients are
extremely weak, i.e., variation in total intensity averages only
~5-10 nT/km and in inclination only ~0.010 o /km. The
weakness of the magnetic gradients has several important
implications for the design of animal navigation systems: a) the
sensory mechanism(s) responsible for detection of spatial variation
in the magnetic field must be extraordinarily sensitive (Phillips,
1996). b) magnetic gradients can not be detected directly, but
instead must be derived from a series of “point samples” (i.e.,
isolated measurements that contain no information about the
alignment of the gradient(s) at the measurement site) that are in a
known spatial relationship to one another (Phillips & Deutschlander,
1997), c) the organism must have an independent means of estimating
geographic position within its area of familiarity (e.g., a place
map, bearing map), or a non-map-based system for determining it’s
spatial position (e.g., a path integration system; Wiltschko &
Wiltschko, 2000; 2003), in order to determine the spatial
relationship among measurement sites, d) the organism must be able
to store precise measurements of magnetic field component(s) in
memory to compare the values obtained at different sites within the
spatial array, and e) because an organism must sample spatially to
build up a knowledge of spatial variation in the magnetic field, use
of a gradient magnetic map will be dependent on experience.
That is, there
should be an ontogenetic progression from reliance on familiar
configurations of landmarks (a place or bearing map) and/or path
integration, to reliance on a full blown gradient map (Wiltschko &
Wiltschko, 2000; 2003).
(2) Due to difference in the iron
content of the underlying rock layers, local gradients in the
magnetic field often differ in direction and/or steepness from the
regional gradient. These local irregularities in the magnetic field
will introduce large errors in map estimates if extrapolation of
the local gradients is used to estimate geographic position after
long distance displacement. As a consequence, a gradient map may
be used at some (but not all) sites for short distance movements
within the range of the local gradients (< 5-10 km). A gradient
magnetic map may also be used for movements over long distances
(>50-75 km) where the regional gradient predominates. For such a
large-scale map, the spatial variation that occurs at distances of
less than ~ 50-75 km (i.e., variation due to local gradients) would
constitute noise. Consequently, such a large-scale magnetic map
could only be used to locate an area 50-75 km in diameter,
rather than a specific site within this area (Phillips, 1996;
Walker 2002). At intermediate distances of from 10-20 km to 50-75 km,
where the contribution of local and regional spatial variation is
similar in magnitude, there is unlikely to be a reliable spatial
signal that could used to provide a meaningful estimate of
geographic position. Therefore, the geomagnetic field is likely to
be used either for a short-distance, high-resolution map or for a
long-distance, low-resolution map, but not for a map that functions at
intermediate distances (Figure 2). Consequently, animals that navigate over
long distances may be forced to rely on different sources of map
information
and/or different types of spatial information (place or compass vs.
gradient maps) for different scales of movement.
(3) Regular temporal variation in
the magnetic field that tends to be greatest during the day light hours could introduce
significant errors in fine scale map estimates. For animals that
use the magnetic field for a short distance (high resolution) map,
therefore, strategies such as averaging multiple measurements over
extended periods of time and/or taking measurements at night when
temporal variation in the magnetic field is minimal may be necessary
to minimize this source of error (Rodda, 1984; Phillips, 1996; Diego-Rasilla
& Phillips, submitted).
(4) Magnetic storms produce large
and unpredictable fluctuations in the magnetic field that could
introduce large errors in estimates of map position, especially in
animals using a short-distance, high resolution map. One way
to minimize such errors would be to use characteristics of magnetic storm activity, e.g., rapid
fluctuations in the geomagnetic field that occur during magnetic
storms, to avoid taking map measurements when the field is unstable
(Phillips, 1996).
(5) Finally, long term variation in
the magnetic field could introduce systematic errors when long
periods of time separate displacement into unfamiliar territory and
the return back to the origin of that displacement, e.g., intervals
of several years that occur in some species between juvenile
dispersal from a natal site, and the return migration of adults to
the natal site to breed (Courtillot et. al, 1997). Strategies such
as taking measurements immediately before and after displacements
(or during the nights immediately before and after displacement) to
minimize the effects of temporal variation, and/or factoring out the
regular component of such variation based on measurements obtained
during intervals between bouts of movement would be necessary to
prevent map coordinates from “drifting” over time due to such long
term changes.
Despite these
very real difficulties, there is a growing body of evidence for the
use of a magnetic map for both short-distance and long-distance
homing. “Route-deprivation” studies
of homing orientation by newts (Phillips, 1986a; Phillips et. al.
1995), juvenile alligators (Rodda, 1984), sea turtles (Lohmann et.
al, 2004) and homing pigeons (Walcott & Schmidt-Koenig, 1973; Boles
& Lohmann, 2003b) show that these animals are able to
use map information obtained at the testing site to determine
geographic ("map") position. Newts provide an especially interesting case.
As discussed previously, in woodland habitats where newts occur,
turbulence in the understory eliminates any consistent relationship
between prevailing wind direction and the direction of air currents
at ground level (Hutschison &
Hicks 1985; Baldocchi 1989), thus eliminating any possibility
that newts use wind-borne odors to learn the distribution of distant
odor sources. The magnetic field, therefore, appears to be the only
potential source of gradient map information available to newts, and
other similar organisms, that live in this type of habitat.
Nevertheless, showing magnetic field effects on homing orientation
is not sufficient to establish the use of a magnetic map. Magnetic
compass (as opposed to map) cues have been shown to play a role in
two important aspects of homing. (1) Young inexperienced
individuals may rely on the magnetic compass to determine
displacement direction (i.e., path integration; Wiltschko &
Wiltschko, 2000; 2003) before they have sufficient experience to
learn the distribution of map cues. (2) Magnetic compass
information may be used for the compass component of homing, e.g.,
when cloud cover restricts access to celestial compass cues (Ioale,
1984; Diego-Rasilla et. al, 2005).
Two lines
of evidence indicate that, in addition to providing compass
information, the magnetic field is an important source of map
information: (1) amphibians and birds have two distinct
magnetoreception mechanisms, one that provides compass information
and one that appears to play a specialized role in the map component
of homing, and (2) under some conditions, experienced adult
amphibians, reptiles and birds respond to small magnetic field
changes as if they have been displaced to a new location (“simulated
magnetic displacements”).
VI.
Dual Magnetic Systems
Magnetic compass orientation in amphibians and birds has been shown
to be sensitive to the presence and wavelength of light (Phillips
& Borland, 1992a; 1992b; Wiltschko et. al,
1993; Wiltschko & Wiltschko, 1995b: Wiltschko & Wiltschko, 1998v; Wiltschko
& Wiltschko, 1999; Wiltschko & Wiltschko 2001; Muheim et. al, 2002),
consistent with theoretical models of the mechanism
of magnetoreception that implicates a photoexcited radical pair
reaction occurring in a specialized photoreceptor (Ritz, et. al,
2000). Both frogs and salamanders trained to exhibit shoreward
orientation under natural light oriented in the trained direction
when tested under full spectrum or short-wavelength (<450 nm) light,
but exhibited 90o
shifted orientation under wavelengths of light > 500 nm
(Phillips & Borland 1992; Freake et. al, 2005). Control
experiments in which newts were trained under wavelengths >
500 nm and tested under either full spectrum or long-wavelength
light indicate that the 90o shift results from a
direction effect of light on the underlying magnetoreception
mechanism (Phillips & Borland, 1992). In newts, the 90o shift is the result of an
antagonistic interaction between short-wavelength (< 450 nm)
and long-wavelength (> 500 nm) inputs mediated by extraocular
photoreceptors located in or near the pineal organ (Phillips &
Borland, 1992; Deutschlander et. al, 1999). Magnetic compass
orientation in birds shows a complex dependence on the wavelength
and intensity of light (Wiltschko & Wiltschko, 2001). Unlike
amphibians, the light-dependent magnetic compass is mediated by
photoreceptors in the retina; as found in a number of visual
mechanisms in birds (e.g., Clayton & Krebbs, 1994), the
light-dependent magnetic compass is strongly lateralized, involving
only the right retina (Wiltschko et. al, 2002b, Wiltschko et. al,
2003). Recent experiments showing effects of low-level radio
frequency radiation on magnetic compass orientation in birds (Ritz
et. al, 2004) are consistent with the energy state transitions
predicted to occur in a mechanism involving a photo-excited radical
pair reaction (Ritz et. al, 2000).
In
addition to the light-dependent magnetic compass, both amphibians and
birds have a second, non-light-dependent mechanism utilizing a
permanent magnetic material that is most likely biogenic magnetite
(Munro et. al, 1997a, Munro et. al, 1997b; Wiltschko et. al, 2002a;
Phillips et. al, 2002a). In birds, the magnetite-based receptor
appears to be associated with the trigeminal nerve system (Beason &
Semm, 1987, Fleissner et. al, 2003). A similar system has been
characterized in salmonid fish, although its role in behavior remains
to be established (Walker et. al, 1997). The magnetite-based
receptor in birds and amphibians is not involved in magnetic compass
orientation, but instead is brought “on line” when these animals
navigate using map-based cues (Phillips, 1986; Phillips & Borland,
1994; Beason & Semm, 1996; Munro et. al, 1997a; 1997b; Beason et.
al, 1997; Phillips et. al, 2003). In birds, evidence for a
magnetite-based receptor’s involvement in the navigational map has come
from pulse remagnetization experiments, in
which animals are exposed to a rapid,
high intensity magnetic pulse that is strong enough to remagnetize
single domain magnetite particles, but with no lasting effects on a
photoreceptor-based mechanism involving a radical pair mechanism.
Munro
et al. (1997a;
1997b)
found that pulse remagnetization altered the migratory orientation
of experienced adult birds that navigate using both map and compass
information, but not that of naïve, young birds that undertake their
first migration using only compass information (Perdeck, 1958).
Similarly, pulse remagnetization was found to affect the initial
orientation of homing pigeons, but only at sites > 80 km from
the home loft (Beason, et. al, 1997 and see Figure 2). The absence of an effect on
pigeons released at shorter distances points to the use of a large
scale, low resolution magnetic map.
 |
|
|
Figure 5. Effects of small changes in
magnetic inclination on homing orientation of Eastern
red-spotted newts (data from Fischer et al. 2001, Phillips
et al. 2002). Magnetic bearings pooled from newts
tested in one of four horizontal alignments of the magnetic
field (magnetic North = north, east, west or south).
Values at the left indicate the changes in inclination to
which the newts were exposed relative to the ambient value
at the testing site (controls). Changes in inclination
were made with little or no change in the total intensity.
Newts were collected from ponds located ~42 km
south-southwest of the testing site (home direction = 207o).
Magnetic inclination at the “home ponds” was approximately
0.17o
less than the inclination at the testing site. mN =
magnetic north. |
|
Experiments with
newts (Fischer et. al, 2001; Phillips et. al, 2002b), sea turtles (Lohmann
et. al, 2004) and migratory birds (Fischer et. al, 2003) have been
carried out to investigate the magnetic field’s role in providing
gradient map information. In these experiments, subjects were
exposed to different values of one or more magnetic field components
similar to those found at locations north and south of the testing
site (“simulated magnetic displacements”). Both newts and sea
turtles exhibited opposite directions of orientation corresponding
to the relative positions of the simulated magnetic map
coordinates.
In initial experiments with
newts, the inclination of the magnetic field was changed by ± 2°
Figure 5a,e). In subsequent experiments, smaller changes in
the magnetic field inclination were used to “titrate” the home
value. Consistent with the magnetic map hypothesis, reversal
of the newts’ homing orientation bracketed the home value of
magnetic inclination (Figure 5b,d) and newts exposed to the home
value of inclination failed to show a consistent direction of
orientation (Figure 5c). To rule out the possibility that the
changes in magnetic inclination affected the magnetic compass, newts
exhibiting shoreward magnetic compass orientation, which does not
require map information, were exposed to ± 2° changes in inclination
(Fischer et. al, 2001). There was no effect on shoreward
magnetic compass orientation, indicating that the magnetic compass
was unaffected.
Simulated
magnetic displacement studies of Tasmanian silvereyes also point to
a possible role of the magnetic field in providing map information,
although the findings are less clear than those observed in newts
and sea turtles. During the fall migration, Fischer et al. (2003)
captured adult silvereyes en route from Tasmania northward
along the southeast coast of Australia. In initial baseline
tests, carried out in the ambient field of Armidale, NSW, silvereyes
oriented in a seasonally appropriate north-northeasterly direction.
The silvereyes were then divided into two groups and exposed to values of magnetic field intensity and
inclination that would normally be encountered to the south
(simulated south displacement; SimS) and to the north (simulated
north displacement; SimN) of the testing site. The northern values
correspond to locations either within (intensity), or to the north
of (inclination), the silvereye’s winter range. The orientation of
the two groups of birds was then tested on alternate days to obtain
5-6 directional responses from each bird. Birds exposed to the simS
displacement continued to show northeasterly magnetic orientation
similar to their control responses, while birds exposed to the simN
displacement failed to show a significant direction of orientation
and differed significantly from their control responses, as well as
from the responses of the birds exposed to the simS condition
(Fisher, et. al, 2003).
 |
|
|
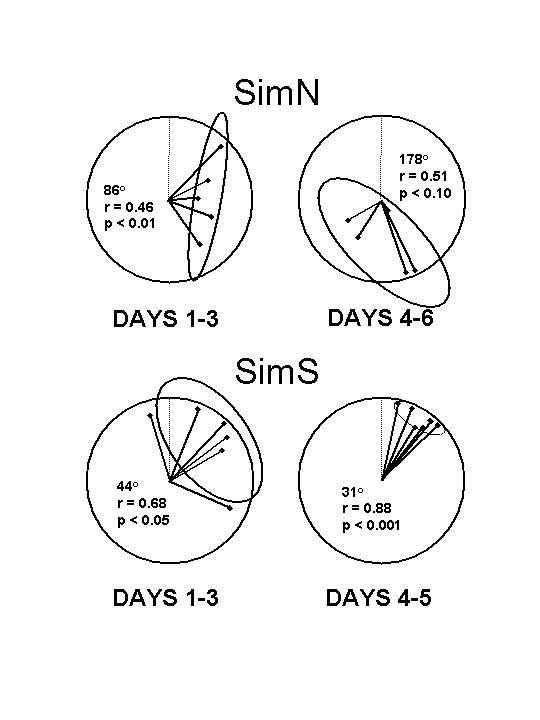
Figure 6: Fall migratory orientation of
adult Tasmanian silvereye tested in orientation funnels in Armidale, NSW, approximately half way along their northward
migratory path from Tasmania along the eastern coast of
Australia (data from Fischer et. al, 2003). SimN birds
were exposed to a 12% decrease in magnetic vertical
intensity that also produced changes in changes in
inclination and total intensity. SimS birds were exposed to
corresponding increases in vertical intensity, total
intensity and inclination. Each vector indicates the mean
direction of orientation of an individual bird over three nights
(left diagrams, upper right diagram) or two nights (lower
right diagram) of testing. Each diagram shows the 95%
confidence ellipse calculated by the Hotelling’s test (Batschelet,
1981). |
|
Fischer et
al. (2003) discussed several possible explanations for the failure of
birds in the SimN condition to exhibit a consistent direction of
orientation. However, a recent reanalysis of these data suggests
that rather than a failure to orient (or a failure of individual
birds to agree on a single direction of orientation), birds in the
SimN condition may have exhibited a two stage response. Figure
6
shows the orientation of birds in the SimS and SimN conditions
during the first three days (Figure 6, left diagrams) and the second
2-3 days (Figure 6, right diagrams) of testing. There was no
difference in the orientation of birds in the SimS condition during
these two time periods (Figure 6, bottom diagrams; o>0.10,
Hotelling's two-sample test). However, birds in
the SimN condition oriented to the east during the first three
nights (Figure 6, top left diagram; p<0.05, Hotelling's one-sample
test), and then exhibited a significant
shift in the direction of orientation (p<0.05, Hotelling's
two-sample test), resulting in a directional
tendency (p<0.10) opposite that of the birds in the SimS birds (Figure
6, bottom
right diagram).
Mouritsen (2003) proposed that
migratory birds may use a unicoordinate map to determine
north-south position, and rely on major topographic features
(rather than a second map coordinate) to determine their east-west
position. In southeastern Australia, silvereyes migrate along a
narrow corridor parallel to the coastline that runs roughly
north-northeast/south-southwest. Consistent with Mouritsen’s
suggestion, the tendency of birds in the SimN condition to fly to
the east would have enabled them to locate the coastline and,
therefore, to determine their east-west position. The subsequent
tendency of the birds to orient in a southerly direction, i.e.,
opposite that of birds in the SimS condition, is consistent with the
birds interpreting their geographic position as being to the north
of their winter range. In other words, these findings suggest that
the birds may be able to derive their north-south
position from the geomagnetic field.
Consistent
with a map effect, more recent experiments using somewhat larger
magnetic field changes have found an affect of the SimN (but not SimS)
condition on the orientation of experienced adult birds, but not that
of naïve young birds captured on the breeding grounds shortly after
fledging and prior to their first migration (Deutschlander et. al, in
preparation). Birds migrating for the first time rely on a
“vector strategy” that combines compass information with a temporal
program (Perdeck, 1958; Berthold, 1990; Berthold, 1991), rather than
true navigation that requires both a map and compass. Other than the
use of map information, the physiological state of young inexperienced
and experienced adult migrants appears to be quite similar (increased
fat reserves, nocturnal restlessness, etc.). Consequently, these
findings suggest that, rather than an effect on the compass, the
response of experienced birds in the SimN condition is due to an effect
on the map.
Can the findings of the simulated
magnetic displacements be explained by an effect on a component of
animals’ navigation system other than the map? In these experiments, subjects were
exposed to a single magnetic field (equivalent to a “point sample”
discussed earlier) and, thus, were unlikely to interpret the altered
magnetic field as a familiar magnetic “landmark” or reference point;
use of magnetic landmarks would require recognition of unique
spatially varying features of local magnetic topography (i.e.,
directions and rates of change in one or more magnetic field
components; Walker, et. al, 2002). Moreover, subjects in the simulated
magnetic displacement experiments were exposed to different magnetic
field conditions at the same site, so other potential map cues such
as odors were held unchanged. Consequently, the findings of these
experiments cannot be explained by assuming that the altered magnetic
fields caused the experimental subjects to attend to
non-magnetic (e.g., olfactory) map cues. The most likely
explanation for the findings of the magnetic displacement
experiments, therefore, is an effect on a magnetic gradient map.
A major unresolved issue is
whether short-distance migrants like newts, and/or long distance
migrants like sea turtles and many species of birds, have unicoordinate or bicoordinate maps. Simulated
magnetic displacement experiments with newts, sea turtles and migratory
birds have provided evidence for the use of the magnetic field to
derive a map coordinate that provides information about geographic
position along roughly the north-south axis. Regional gradients
in magnetic field components such as inclination and intensity run
more-or-less north-south and, thus, are well suited to provide
information about geographic position along this axis. It remains
to be determined whether these animals are able to determine their
geographic position along a second, non-parallel (east-west) axis.
If these animals are able to use a
second map coordinate, what might this coordinate be? In many parts
of the world, large-scale variation in magnetic declination (the
angle between magnetic and geographic north) varies along a gradient
that is roughly orthogonal to the gradients of intensity and
inclination and, thus, provides a possible second map coordinate.
Birds have been proposed to derive geographic North as a reference
for calibration of their compass systems by averaging the positions
of the band of maximum skylight polarization at sunrise and sunset
(Phillips & Waldvogel, 1982; 1988). It remains to be determined
whether measurements of magnetic declination using such a reference
system would be accurate enough for a large-scale
map. Nevertheless, for long-distance migrants like silvereyes, a
second map coordinate derived from magnetic declination remains a
possibility. For example, silvereyes migrating from Tasmania
northward along the coast of eastern Australia travel in a corridor
approximately 200 km wide. Magnetic declination changes by about 2
degrees over this distance. Since the gradient of magnetic
declination is roughly perpendicular to the gradients of intensity
and inclination, silvereyes provide an excellent model system in
which to investigate the possibility of a bicoordinate magnetic
map. Use of a bicoordinate magnetic map is not ruled out by the
experiments described earlier in which silvereyes appeared use an
alternative strategy (“fly east until you encounter the coastline”)
to determine east-west position (Figure 6). The birds in these
experiments were housed and tested indoors without access to
celestial cues and, thus, could not have used a reference system
based on celestial polarized patterns to obtain measurements of
magnetic declination (see earlier discussion).
Bicoordinate
navigation is even more problematic for animals that require short
distance, high resolution map information (i.e., for movents over
distance <5-10 km). At such short distances, it is unlikely
that magnetic declination could to be used to derive a second map
coordinate, since the required accuracy would be roughly 100 times
greater than that required by (e.g.) a migratory bird, and the forest
canopy would limit the access of newts and other inhabitants of the
forest floor to celestial cues such as polarized light patterns that
could be used to derive a geographic north reference (Phillips &
Waldvogel, 1988). An alternative possibility is that local
variation in the magnetic field results in non-parallel gradients of
components such as inclination and intensity that do not require an
independent directional reference. However, local gradients
suitable for bicoordinate navigation are likely to be present at some
sites, but not at others (Phillips, 1996). If so, a bicoordinate
magnetic map cannot provide a universal solution to the problem of
navigation over small spatial scales. An interesting question is
whether newts, and other organisms that require short-range, high
resolution map information, are be found at found at hgher population
densities in areas where local magnetic field gradients are suitable
for bicoordinate navigation (S.C. Borland, personal communication).
We have argued for a fundamental
distinction between local “place” maps (and related maps that
involve familiar reference sites, including bearing maps), and
larger scale gradient maps that can be extrapolated beyond an
individual’s area of familiarity. Not only are these two categories
of maps likely to involve different types of sensory information,
but also different processing mechanisms and, consequently,
different populations of neurons in the central nervous system.
Contrary to a number of recent authors (Jacobs & Schenk, 2003; Walraff,
2004; Bingman et. al, this volume), we also conclude that
there is no compelling evidence for the involvement of olfactory
cues in a large-scale gradient map. In contrast, a compelling case
is emerging for the use of magnetic cues to derive at least one map
coordinate. Both amphibians and birds have a magnetoreception
mechanism distinct from the magnetic compass that appears to play a
specialized role in the map component of homing. Simulated magnetic
displacement experiments have provided evidence that amphibians,
reptiles and birds (see also recent findings from spiny lobsters;
Boles & Lohmann, 2003) will, at least under some conditions,
interpret small changes in the magnetic field as changes in
geographic position. Nevertheless, magnetic navigation does not
provide a universal solution to the problem of map-based homing,
i.e., the magnetic field does not provide useful map information at
all sites or at all spatial scales.
The
emerging picture of animal navigation
systems that is one of a patchwork of mechanisms that vary in
utility depending on local environment (e.g.,
the characteristics of local gradients), range of movement, and
individual experience. Map information involving different sensory
modalities may be involved, including both olfactory and magnetic
cues, as well as both map-based and route-based mechanisms for
deriving spatial position at unfamiliar locations. Furthermore,
both map-based and route-based sources of spatial information are
likely to be replaced by large scale place or bearing maps as
increased familiarity produces qualitative changes in the processing
and neural representation of spatial information (Jacobs & Schenk,
2003). Further work is needed to determine the range over which
different navigational mechanisms operate (e.g., Beason et. al, 1997;
Schmidt-Koenig, 1966; 1970), whether gradient maps provide
unicoordinate or bicoordinate information, and the extent to which
olfactory cues are involved in
providing map information as opposed to distinguishing home from non-home, recognizing familiar sites, and
triggering recall of stored map and compass reference information.
Acknowledgements.
Support was
provided by National Science Foundation grants IBN02-16957 and IBN04
25712 (to JBP), and the Swiss National Science Foundation
(postdoctoral fellowship) to R.M.
Baldicinni NE, Benvenuti S, Fiaschi
V, Papi F (1975). Pigeon
navigation: Effects of wind deflection at home cage on homing
behavior. J. Comp. Physiol. A 99, 177-186.
Beason RC,
Semm P
(1987). Magnetic responses of
the trigeminal nerve system of the Bobolink Dolichonyx oryzivorus.
Neurosc.Lett. 80,
229-234.
Beason RC, Semm
P (1996). Does the avian ophthalmic nerve carry magnetic
navigational information? J. Exp. Biol. 199, 1241-1244.
Beason RC,
Wiltschko R,
Wiltschko W
(1997). Pigeon homing: effects
of magnetic pulses on initial orientation.
The Auk 114[3],
405-415.
Berthold P
(1990). Spatiotemporal programs
and genetics of orientation.
Experientia 46,
363-371.
Berthold P
(1991). Genetic control of
migratory behaviour in birds.
TREE 6,
254-257.
Bingman VP,
Able KP,
Siegel JJ
(1999). Hippocampal lesions do
not impair the geomagnetic orientation of migratory Savannah
Sparrows. J.Comp.Physiol.A 185,
577-581.
Bingman VP,
Gagliardo A,
Ioalè P
(1996). Hippocampal
participation in the sun compass orientation of phase-shifted homing
pigeons. J.Comp.Physiol.A 179,
695-702.
Bletz H,
Weindler P,
Wiltschko R,
Wiltschko W
(1996). The magnetic field as
reference for the innate migratory direction in Blackcaps (Sylvia
atricapilla).
Naturwissenschaften 83,
430-432.
Baldocchi DD (1989). Turbulent transfer in a
deciduous forest. Tree physiology. 5, 357-377.
Boles LC,
Lohmann KJ
(2003). True navigation and
magnetic maps in spiny lobsters.
Nature 421,
60-63.
Brines ML,
Gould JL
(1982). Skylight polarization
patterns and animal orientation.
J.Exp.Biol. 96,
69-91.
Burt T, Holland R, Guilford T
(1997). Further evidence for
visual landmarks involvement in the pigeons’s familiar area map.
Anim. Behav. 53: 1203-1209.
Casini G. Fontanese G, Bingman V,
Jones TJ, Gagliardo A, Ioale P, Bagnoli P
(1997). The neuroethology of cognitive maps: Contributions from
research on the hippocampus and homing pigeon navigation. Arch.
Ital. Biol. 135: 73-92.
Clayton NS, Krebbs JR
(1994). Memory for spatial and
object-specific cues in food storing birds. J. Comp. Physiol. 174,
371-379.
Courtillot V,
Hulot G,
Alexandrescu M,
le Mouël J-L,
Kirschvink JL
(1997). Sensitivity and
evolution of sea-turtle magnetoreception: observations, modelling
and constraints from geomagnetic secular variation.
Terra Nova 9,
203-207.
Deutschlander, ME,
Borland, SC, Phillips JB (1999).
Extraocular magnetic compass in newts. Nature 400, 324-325.
Diego-Rasilla FJ,
Luengo, RM, Phillips JB
(2005). Magnetic compass mediates nocturnal homing by the alpine
newt, Triturus alpestris. Behav. Ecol. Sociobiol.
58, 361-365 .
Dittman AH, Quinn TP
(1996). Homing in pacific salmon: Mechanisms and ecological
basis. J. Exp. Biol. 199, 83-91.
Emlen ST
(1970). Celestial rotation: its
importance in the development of migratory orientation.
Science 170,
1198-1201.
Emlen ST
(1975). Migration: Orientation
and Navigation. Farner DS
and King JR.
Avian Biology, Vol. V.
[3],
129-219. New York,
Academic Press.
Fischer JH,
Freake MJ,
Borland SC,
Phillips JB
(2001). Evidence for the use of
a magnetic map by an amphibian.
Anim.Behav. 62,
1-10.
Fischer JH,
Munro U,
Phillips JB
(2003). Magnetic navigation by
an avian migrant? Berthold P,
Gwinner E,
and Sonnenschein E.
Avian Migration.
423-432. Heidelberg, New York,
Springer-Verlag.
Fleissner G,
Holtkamp-Rötzler E,
Hanzlik M,
Winklhofer M,
Fleissner G,
Petersen N,
Wiltschko W
(2003). Ultrastructural analysis
of a putative magnetoreceptor in the beak of homing pigeons.
J.Comp.Neurol. 458,
350-360.
Foa A, Bagnoli P, Giongo F (1986). Homing pigeons subjected
to section of the anterior commissure can build up two olfactory
maps in the deflector lofts. J. Comp. Physiol. A 159, 465-472.
Freake MJ, Phillips JB (2005). Light-dependent shift in
bullfrog tadpole magnetic compass orientation: evidence for a
common magnetoreception mechanism in anuran and urodele amphibians.
Ethology 111, 241-254.
Gagliardo A, Ioale P, Bingman VP
(1999). Homing in pigeons:
The role of the hippocampus formation in the representation of
landmarks used for navigation. J. Neurosci. 19, 311-315.
Gagliardo A, Ioale P, Odetti F, Kahn
MC, Bingman VP (2004).
Hippocampal lesions do not dirupt navigational map retention in
homing pigeons under conditions when amp acquisition is hippocampal
dependent. Behav. Brain Res. 153, 35-42.
Gagliardo A, Mazzotto M, Bingman V
(1997). Piriform cortex
ablations block navigational map learning in homing pigeons.
Behav. Brain Res. 86,
143-148.
Ganzhorn, JU (1990).
Towards the map of the homing pigeon.
Anim. Behav. 40, 65-78.
Ganzhorn, JU
(1992). Geographical patterns in the initial orientation of homing
pigeons in upstate New York. Anim. Behav. 44, 931-941.
Ganzhorn, JU
(1995). Patterns of air pollution as model for the physical basis
for olfactory navigation in pigeon homing.
J. Ornithol. 136, 159-165.
Ganzhorn JU,
Schmidt-Koenig K
(1990). Initial orientation of
homing pigeons around Tübingen, Elzen, Rvd, Schuchmann, K.-L, &
Schmidt-Koenig, K), Current Topics in Avian Biology, J. Orn.
Sonderheft,
pp. 305-309.
Gould JL
(1982). The map sense of pigeons.
Nature 296,
205-211.
Gould JL
(1985). Are animal maps
magnetic? Kirschvink JL,
Jones DS,
and McFadden BJ.
Magnetite Biomineralization and
Magnetoreception in Organisms: a New Biomagnetism.
257-268. New York London,
Plenum Press.
Grant D, Anderson O, Twitty V (1968). Homing orientation by olfaction in newts Taricha
rivularis. Science 160, 1354-1355.
Griffin DR
(1952). Bird Navigation.
Biol.Rev.Camb.Philos.Soc. 27,
359-400.
Guilford T,
Gagliardo A,
Chappell J,
Bonadonna F,
Burt T,
Holland R
(1998). Homing pigeons use
olfactory cues for navigation in England.
J.Exp.Biol. 201,
895-900.
Gwinner E,
Wiltschko W
(1978). Endogenously controlled
changes in the migratory direction of garden warbler, Sylvia borin. J.Comp.Physiol. 125,
267-273.
Hays GC,
Åkesson S,
Broderick AC,
Glen F,
Godley BJ,
Papi F,
Luschi P
(2003). Island-finding ability
of marine turtles. Proc.R.Soc.Lond.B
270 (supplement), S5-S7.
Helbig AJ
(1990). Depolarization of natural skylight disrupts orientation of
an avian nocturnal migrant. Experientia 46, 755-758.
Helbig AJ
(1992). Ontogenetic stability of
inherited migratory directions in a nocturnal bird migrant:
comparison between the first and second year of life.
Ethol.Ecol.Evol. 4,
375-388.
Helbig AJ
(1996). Genetic basis, mode of
inheritance and evolutionary changes of migratory directions in
paleaarctic warblers (Aves: Sylviidae).
J.Exp.Biol. 199[1],
49-55.
Hutchison BA, Hicks DD
(1985). The Forest-atmosphere
interaction: proceedings of the Forest Environmental Measurements
Conference, Oakridge Tenessee. Dordrecht: D. Reidel.
Ioale P
(1984). Magnets and pigeon
orientation. Monit. Zool. Ital. 18: 347-358.
Ioale P, Gagliardo A, Bingman V
(2000). Hippocampal participation in navigational map learning in
young homing pigeons is dependent on training experience. Euro. J.
Neurosci. 12: 742-750.
Jacobs, LF & F Schenk
(2003). Unpacking the cognitive map: The parallel map theory of
hippocampal function. Psych. Rev. 110, 285-315.
Johnsen PB, Hasler AD
(1980). The use of chemical cues in
the upstream migration of coho salmon. J. Fish Biol. 17: 67-74.
Joly P, Miaud C
(1989). Fidelity to the breeding site in the alpine newt Triturus
alpestris.
Behavioural Processes 19: 47-56
Joly P, Miaud C
(1990). How does a newt finds its
pond? The role of chemical cues in migrating newts. Ethol. Ecol.
Evol. 5, 447-455.
Kiepenheuer J
(1978). Pigeon homing: a repetition of the deflector loft
experiment. Behav. Ecol. Sociobiol. 3, 393-395.
Kiepenheuer J
(1979). Pigeon homing: Deprivation of olfactory information does
not affect the deflector loft effect. Behav. Ecol. Sociobiol. 6,
11-22.
Kiepenheuer J
(1982). Pigeon orientation: A preliminary evaluation of factors
involved or not involved in the deflector loft effect.
Papi F, Wallraff HG, Avian
navigation, 203-210, Berlin, Springer-Verlag.
Kiepenheuer J
(1985). Can pigeons be fooled about the actual release site
position by presenting them information from another site. Behav.
Ecol. Sociobiol. 18, 75-82.
Lohmann KJ,
Cain SD,
Dodge SA,
Lohmann CMF
(2001). Regional magnetic fields
as navigational markers for Sea Turtles.
Science 294,
364-366.
Lohmann KJ, Lohmann CMF
(1994). Detection of magnetic
inclination angle by sea turtles: A possible mechanism for
determining latitude. J.Exp.Biol. 194,
23-32.
Lohmann KJ, Lohmann CMF
(1996). Orientation and open-sea
navigation in sea turtles.
J.Exp.Biol. 199,
73-81.
Lohmann KJ,
Lohmann CMF,
Ehrhart LM,
Bagley DA,
Swing T
(2004). Animal behaviour:
Geomagnetic map used in sea-turtle navigation
. Nature 428,
909-910.
Matthews GVT
(1963). The orientation of pigeons as affected by the learning of
landmarks and by the distance of displacement. Anim. Behav. 11,
310-317.
Moore BR
(1980). Is the homing pigeon's
mag geomagnetic? Nature 285,
69-70.
Moore FR
(1987). Sunset and the
orientation behaviour of migrating birds.
Biol Rev 62,
65-86.
Mora CV, Davidson M, Wild JM, Walker
MM (2004). Magnetoreception
and its trigeminal mediation in the homing pigeon. Nature
432,508-511.
Mouritsen H
(2003). Spatiotemporal orientation strategies of long-distance
migrants. Berthold P,
Gwinner E, Sonnenschein E, Avian Migration, 493-513, Berlin:
Springer Verlag.
Muheim R,
Bäckman J,
Åkesson S
(2002). Magnetic compass
orientation in European robins is dependent on both wavelength and
intensity of light. J.Exp.Biol. 205[24],
3845-3856.
Muheim R, Moore FR, Phillips JB.
Calibration of magnetic and celestial compass cues in migratory
birdsppa review of cue-conflict experiments. J. Exp. Biol.
(submitted).
Munro U,
Munro JA,
Phillips JB
(1997). Evidence for a
magnetite-based navigational 'map' in birds.
Naturwissenschaften 84,
26-28.
Munro U,
Munro JA,
Phillips JB,
Wiltschko W
(1997). Effect of wavelength of
light and pulse magnetization on different magnetoreception systems
in a migratory bird. Austral.J.Zool. 45,
189-198.
Papi F (1990). Olfactory navigation in birds. Experientia 46, 352-362.
Perdeck AC
(1958). Two types of orientation
in migrating Starlings Sturnus vulgaris and Chaffinches Fringilla
coelebs as revealed by displacement experiments.
Ardea 46,
1-37.
Phillips JB
(1986a). Magnetic compass
orientation in the Eastern red-spotted newt Notophthalmus
viridescens. J. Comp. Physiol. A 158,
103-109.
Phillips JB
(1986b). Two magnetoreceptor
pathways in a migratory salamander.
Science 233,
765-767.
Phillips JB
(1987). Laboratory studies of
homing orientation in the Eastern red-spotted newt Notophthalmus
viridescens. J.Exp. Biol. 131,
215-229.
Phillips JB
(1996). Magnetic navigation.
J. Theor. Biol. 180,
309-319.
Phillips JB,
Adler K,
Borland SC
(1995). True navigation by an
amphibian. Anim. Behav. 50,
855-858.
Phillips JB,
Borland SC
(1992a). Magnetic compass
orientation is eliminated under near-infrared light in the Eastern
red-spotted newt, Notophthalmus viridescens.
Anim.Behav. 44,
796-797.
Phillips JB,
Borland SC
(1992b). Wavelength-specific
effects of light on magnetic compass orientation of the Eastern
red-spotted newt Notophthalmus viridescens.
Ethol.Ecol.Evol. 4,
33-42.
Phillips JB,
Borland SC,
Freake MJ,
Brassart J,
Kirschvink JL
(2002a). `Fixed-axis' magnetic
orientation by an amphibian: non-shoreward-directed compass
orientation, misdirected homing or positioning a magnetite-based map
detector in a consistent alignment relative to the magnetic field?
J.Exp.Biol. 205[24],
3903-3914.
Phillips JB, Deutschlander ME
(1997). Magnetoreception in terrestrial vertebrates: Implications
for possible mechanisms of EMF interaction with biological systems.
Stevens RG, Andrews LE, Wilson BW, The Melatonin Hypothesis:
Electric Power and the Risk of Breast Cancer, Battelle Press,
Columbus, OH, pp. 111-172.
Phillips JB,
Freake MJ,
Fischer JH,
Borland SC (2002b). Behavioural titration
of a magnetic map coordinate.
J.Comp.Physiol.A 188,
157-160.
Phillips JB,
Moore FR
(1992). Calibration of the sun
compass by sunset polarized light patterns in a migratory bird.
Behav.Ecol.Sociobiol. 31,
189-193.
Phillips JB,
Waldvogel JA
(1982). Reflected light cues
generate the short-term deflector-loft effect.
Papi F
and Wallraff E.
Avian Navigation. 190-202. Berlin,
Springer-Verlag.
Phillips JB,
Waldvogel JA
(1988). Celestial polarized
patterns as a calibration reference for sun compass of homing
pigeons. J.Theor.Biol. 131,
55-67.
Ritz T, Adem S, Schulten K
(2000). A model for photoreceptor-based magnetoreception in
birds. Biophys. J. 78, 707-718.
Ritz T,
Thalau P,
Phillips JB,
Wiltschko R,
Wiltschko W
(2004). Resonance effects
indicate a radical-pair mechanism for avian magnetic compass.
Nature 429[6988 ],
177-180.
Rodda GH
(1984). The orientation and
navigation of juvenile alligators: evidence of magnetic sensitivity.
J.Comp.Physiol.A 154,
649-658.
Rodda GH,
Phillips JB
(1992). Navigational systems
develop along similar lines in amphibians, reptiles and birds.
Ethol.Ecol.Evol. 4,
43-51.
Saint Paul Uv
(1953). Nachweis der
Sonnenorientierung bei nächtlich ziehenden Vögeln.
Behaviour 6,
1-7.
Sauer EGF,
Sauer EM (1960). Star navigation of
nocturnal migrating birds.
Cold Spring Harbor Symposium
Quantitative Biology 25, 463-473.
Schmidt-Koenig K
(1958). Experimentelle Einflussnahme auf die 24-Stunden-Periodik
bei Brieftauben und deren Auswirkungen unter besonderer
Berücksichtigung des Heimfindevermögens. Z. Tierspychol. 15,
301-331.
Schmidt-Koenig K
(1966). Ueber die Entfernung als Parameter bei
derAnfangsorientierung der Brieftaube.
Z. Vergl. Physiol. 52, 33-55.
Schmidt-Koenig K (1970). Entfernung und Heimkehrverhalten derBrieftaube.
Z. Vergl. Physiol. 68, 39-48.
Schmidt-Koenig K
(1979). Avian Orientation and
Navigation. London,
Academic Press.
Schmidt-Koenig K
(1990). The sun compass.
Experientia 46,
336-342.
Sinsch, U.
1990a. Migration and orientation
in anuran amphibians. Etholology Ecology & Evolution, 2,
65-79.
Sinsch, U.
1990b. The orientation
behaviour of three toad species (genus Bufo) displaced from the
breeding site. In:
Biology and Physiology of Amphibians (Ed. by W. Hanke), pp.
73-83. G. Fischer
Verlag, Fortschritte der Zoologie 38.
Skiles DD
(1985). The geomagnetic field:
its nature, history, and biological relevance.
Kirschvink JL,
Jones DS,
and McFadden BJ.
Magnetite Biomineralization and
Magnetoreception in Organisms: a New Biomagnetism.
43-102. New York, London,
Plenum Press.
Walcott C
(1991). Magnetic maps in
pigeons. Berthold P.
Orientation in Birds. 38-51. Basel,
Birkhäuser Verlag.
Walcott,C, Schmidt-Koenig
K (1973).
The effect on pigeon homing of
anesthesia during displacement.
Auk, 90, 281-286.
Waldvogel JA
(1987). Olfactory navigation in
homing pigeons: are the current models atmospherically realistic?
Auk 104, 369-379.
Waldvogel JA, Benvenuti S, Keeton
WT, Papi F (1978). Homing
pigeon orientation influenced by deflected winds at home loft. J.
Comp. Physiol. 128, 297-301.
Waldvogel JA, Phillips JB
(1982). Pigeon Homing: New
experiments involving permanent-resident deflector-loft birds.
Papi F, Wallraff HG,
Avian Navigation, 179-189, Berlin, Springer Verlag.
Waldvogel JA, Phillips JB
(1991). Olfactory cues
perceived at the home loft are not essential for the formation of a
navigational map in pigeons.
J.Exp.Biol. 155,
643-660.
Walker M, Diebel CE, Haugh CV,
Pankhurst PM, Montogmery JC, Green CR
(1997). Structure and function of the
vertebrate magnetic sense. Nature 390, 371-376.
Walker MM,
Dennis TE,
Kirschvink JL (2002). The magnetic sense and
its use in long-distance navigation by animals.
Curr Opin Neurobiol 12
, 735-744.
Wallraff HG
(2004). Avian olfactory navigation: Its empirical foundation and
conceptual basis. Animal Behaviour 67, 189-204.
Wallraff HG, Kiepenheuer J, Neumann
MF, Streng A (1995). Homing
experiments with starlings deprived of the sense of smell. Condor
97: 20-26.
Wehner R
(1998). Navigation in context:
grand theories and basic mechanisms.
J.Avian Biol. 29,
370-386.
Weindler P,
Wiltschko R,
Wiltschko W
(1996). Magnetic information
affects the stellar orientation of young bird migrants.
Nature 383,
158-160.
Wiltschko R
(1996). The function of
olfactory input in pigeon orientation: does it provide navigational
information or play another role? J.Exp.Biol. 199[1],
113-119.
Wiltschko R,
Schöps M,
Kowalski U
(1989). Pigeon homing: wind
exposition determines the importance of olfactory input.
Naturwissenschaften 76,
229-231.
Wiltschko R,
Wiltschko W
(1995). Magnetic Orientation in
Animals.
Berlin, Springer-Verlag.
Wiltschko R,
Wiltschko W
(1998). Pigeon homing: effect
of various wavelengths of light during displacement.
Naturwissenschaften 85,
164-167.
Wiltschko R, Wiltschko W
(2000). A strategy for beginners! Reply to Wallraff (2000). Anim.
Behav. 60, F37-F43.
Wiltschko R, Wiltschko W
(2003). Avian navigation: from historical to modern concepts.
Anim. Behav. 65, 257-272.
Wiltschko W,
Daum P,
Fergenbauer-Kimmel A,
Wiltschko R
(1987). The development of the
star compass in Garden Warblers Sylvia borin.
Ethology 74,
285-292.
Wiltschko W,
Munro U,
Ford H,
Wiltschko R
(1993). Red light disrupts
magnetic orientation of migratory birds.
Nature 364,
525-527.
Wiltschko W,
Munro U,
Ford H,
Wiltschko R
(2003). Lateralization of
magnetic compass orientation in silvereyes, Zosterops l. lateralis.
Austral.J.Zool. 51,
597-602.
Wiltschko W,
Munro U,
Wiltschko R,
Kirschvink JL
(2002). Magnetite-based
magnetoreception in birds: the effect of a biasing field and a pulse
on migratory behavior. J.Exp.Biol. 205[19],
3031-3037.
Wiltschko W,
Traudt J,
Güntürkün O,
Prior H,
Wiltschko R
(2002). Lateralization of
magnetic compass orientation in a migratory bird.
Nature 419,
467-470.
Wiltschko W,
Wiltschko R
(1972). Magnetic compass of
European Robins. Science 176,
62-64.
Wiltschko W,
Wiltschko R
(1995). Migratory orientation
of European Robins is affected by the wavelength of light as well as
by a magnetic pulse. J.Comp.Physiol.A 177,
363-369.
Wiltschko W,
Wiltschko R
(1999). The effect of yellow
and blue light on magnetic compass orientation in European Robins
Erithacus rubecula. J.Comp.Physiol.A 184,
295-299.
Wiltschko W,
Wiltschko R
(2001). Light-dependent
magnetoreception in birds: the behaviour of European robins,
Erithacus rubecula, under monochromatic light of various wavelengths
and intensities. J.Exp.Biol. 204,
3295-3302.
Yeagley HL
(1947). A preliminary study of
a physical basis of bird navigation. Part I. Journal of Applied Physics 18[12],
1035-1063.
Yeagley HL
(1951). A preliminary study of
a physical basis of bird navigation. Part II.
Journal of Applied Physics 22[6],
746-760



