The
extraordinary navigational ability of animals, which enable some
species to carry out remarkably precise long-distance migrations and
homing behavior, has fascinated natural historians for as long as
animal behavior has been of interest. The observation of an arctic
tern (Sterna paradisaea) carrying out a yearly migration
between the arctic regions of the northern and southern hemisphere,
a gray whale (Eschrichtius robustus) migrating between cold
water feeding areas near Alaska and birthing sites around the Baja
peninsula, a loggerhead sea turtle (Caretta caretta)
migrating from feeding areas in the north Atlantic to egg deposition
sites on the coastal beaches in tropical and sub-tropical North
America, and a monarch butterfly (Danaus plexippus) making a
one-way flight from temperate North America to their winter
congregation site in central Mexico can seem mystifying (Figure 1).
In fact, the seemingly routine ability of animals in general to
accurately navigate space nurtures the speculation that the
evolution of spatial cognitive abilities may have also served as
pre-adaptation for other forms of cognition and associated brain
mechanisms (e.g., O’Keefe, 1996). But how do animals navigate? The
goal of this chapter is to review the behavioral mechanisms that are
exploited by animals as they navigate large-scale, environmental
space, as well present some findings related to brain mechanisms
that support this ability. Because of their dramatic spatial
behavior and extensive use as experimental subjects, we will
concentrate our review on birds.
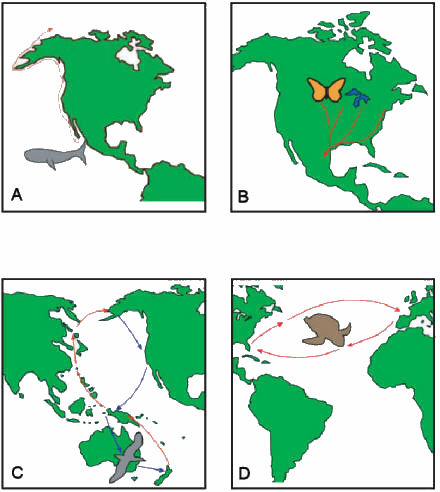 |
Figure 1:
Global migratory paths of four exceptional
navigators: A) gray whale (Eschrichtius robustus);
B) monarch butterfly (Danaus plexippus); C)
arctic tern (Sterna paradisaea); D)
loggerhead sea turtle (Caretta caretta). |
The
ability to polarize space within some directional framework is
essential if animals are to maintain movement in a constant
direction with respect to the environment. Metaphorically, the
challenge is similar to a human navigator needing to use a compass
to identify directions in space and maintain a constant directional
bearing while moving. Animal navigators possess biological compasses
based on their sensitivity to the position of the sun projected on
the horizon, or azimuth, stars and the earth’s magnetic field. These
compass mechanisms, although providing only directional information,
form the basis from which richer, map-like representations of space
can emerge.
Sun Compass. For diurnal animals with sensory access to the sky,
the sun undoubtedly offers the richest source of information to
define compass directions and orient movements in space whether it
is a short-distance flight of a bee navigating between its hive and
a food source or a diurnally migrating swallow making a journey of
several thousand kms. The discovery and properties of the sun
compass in birds were thoroughly investigated by numerous German
researchers in the 1950s and ‘60s (Kramer, 1952, 1959; Hoffmann,
1954; Schmidt-Koenig 1958, 1961). Conceptually, the challenge the
sun presents an animal that wants to maintain, for example, a
southerly bearing is that the position of the sun in the sky changes
during the course of the day. To continue moving south, a bird in
the northern hemisphere would need to keep the easterly sun to its
left in the morning, fly toward the southerly sun at midday and
keep the westerly sun to its right in the evening. The changing
azimuth of the sun across the day needs to be calibrated with
respect to stable compass directions in space. Birds seem to carry
out this conceptually challenging computation effortlessly. They do
so by relying on their internal sense of time, which manifests
itself in the form of endogenous circadian rhythms. Endogenous,
biological circadian rhythms oscillate with a period of about 24
hours and are entrained or calibrated against the light-dark cycle
of the environment. A point in time would correspond with a point in
the cycle of the circadian rhythm. As such, reading off the
circadian rhythm can be used to define time of day and therefore be
used to read off a compass bearing from the sun’s azimuth.
 |
Figure 2:
Video illustration of a homing pigeon experimental
release that could be used to determine the
properties of the sun compass.
Click on figure to start video.
|
How do we know that the temporal
calibration of the sun compass recruits endogenous circadian rhythms
as the time giver? This was elegantly demonstrated in birds (homing
pigeons and starlings) by placing experimental subjects in an
environment where the light-dark cycle was shifted; for example, the
lights in the room would come on at midnight and go off at noon
basically advancing the day of the birds 6 hours relative to the
light-dark cycle of the natural environment. Birds kept in these
conditions for a week or so would experience a shift in their
circadian rhythms; a rhythm would recalibrate to the changed
light-dark cycle such that the circadian rhythm’s morning would
correspond or entrain to lights coming on, which would be midnight
with respect to the natural environment. Imagine now a migratory
bird or homing pigeon that would typically orient south held in the
shifted light-dark cycle for a week. The bird would then be tested
for its orientation, either by letting it fly (see Figure 2 and 3)
or in a cage, during the natural morning when the sun is in the
east. However, for our experimental bird the reading of its
circadian rhythm would indicate that it is noon (remember its
circadian rhythm has been shifted), and you would actually observe
the bird orient not in the desired southerly direction but east
(Figure 3)! Why? The midday sun is in the south, and according to
the bird’s internal rhythm, it is noon and it should fly toward the
sun. But the sun is really in the east during the environmental
morning; therefore movement toward the sun is actually an easterly
movement and the wrong direction. It is this type of clock- or
phase-shift experiment that has demonstrated that birds, and other
animal groups including monarch butterflies (Mouritsen and Frost,
2002), use their internal sense of time to calibrate the movement of
the sun in the sky. This enables them to use the sun as a stable
reference to define compass directions in space.
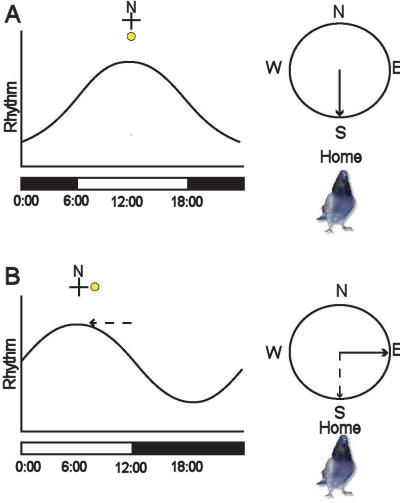 |
Figure 3:
Effect of a phase-shift in the light dark cycle
on the sun compass orientation of homing
pigeons. A. Under natural conditions, the
properties of a circadian rhythm would peak
during midday and be associated with the sun in
the south. A pigeon needing to fly south would
then orient toward the sun at midday. B. After
being held for one week in a room where the
light-dark cycle has been advanced by six hours,
the peak in the circadian rhythm associated with
midday would now occur during the natural
morning. When released now during the natural
morning, the pigeon’s subjective noon, the same
bird needing to fly south would again fly toward
the sun, which would be east! Because of the
change in the circadian rhythm, the peak in the
circadian rhythm previously associated with
midday and the sun in the south would now
actually correspond to environmental morning and
the sun in the east. |
To end
our discussion of the sun compass, it should be mentioned that in
addition to the disc of the sun, birds can also orient to patterns
of skylight polarization derived from the sun. They can do so
because the properties of skylight polarization change predictably
with the changing position of the sun (e.g., Able, 1982). Bird
visual sensitivity to ultraviolet light, like that of bees, may be
important in detecting skylight polarization
Star compass.
The sun is not the only celestial body that can be exploited to
define directions in space. Although nocturnal migrant birds can
and do use the position of the setting sun to orient their
nighttime migrations (Moore, 1987), they can also rely on the
stars. But it is not just any star or cluster of stars that can
be used to guide migration. It is the stars around the axis
point of the night’s sky apparent rotation that are
preferentially relied on (Emlen, 1967). In the Northern
Hemisphere, these would be circumpolar stars like those found in
the constellations of the Big Dipper and Cassiopeia. However,
this star compass has properties different from the sun compass.
For example, orientation to the stars is not time compensated;
phase shifting migrant birds does not alter their migratory
orientation to the stars as it would sun compass orientation. It
is also notable that whereas birds can be trained to use the sun
compass to orient to a food source or other goal unrelated to
migration or homing, orientation by the stars has only been
demonstrated in the context of migration.
Geomagnetic compass. The
sensitivity of birds to visual orientation stimuli is not surprising
given that they have well developed visual systems, and the idea of
a sun and star compass was quickly embraced by researchers. This was
not the case for the now well established behavioral ability of
birds to orient by the earth’s magnetic field. The problem with
celestial cues is that there are times, in some places frequently,
when the sky is obscured by clouds. Lengthy periods of time without
access to celestial orientation cues could substantially compromise
survival and reproduction if birds could not rely on some
alternative compass mechanism. In areas familiar to a bird, known
landmarks could serve as orientation cues. But what about a migrant
flying high over completely unfamiliar terrain? A sensitivity to the
earth’s magnetic field, the central nervous representation of which
still remains poorly understood, is the solution that natural
selection has provided birds for the challenge of compass
orientation without access to celestial cues. The experimental
demonstration of geomagnetic orientation is apparent when either
migrant birds or homing pigeons experience a shift in the ambient
magnetic field lines under conditions when they would rely on
non-visual cues for orientation. Simply, the birds shift their
orientation in parallel with the altered magnetic field. One curious
property of their magnetic compass is that it is not the kind of
compass that people use while hiking; a so-called polarity compass.
Rather, the bird geomagnetic compass is a so-called inclination
compass by which north and south are defined by the angle the
ambient magnetic field lines make with some vertical reference like
gravity (Wiltschko and Wiltschko, 1972).
Ontogeny: the importance of experience. This book is about
spatial cognition, and the compass mechanisms described above would
not usually be considered in discussions of animal cognition. At
first glance they have an innate, reflexive quality that might be of
more interest to an ethologist than a traditional comparative
psychologist. However, as we will present below, spatial behaviors
readily identified as relying on “cognitive” representations are
grounded in these compass mechanisms under field conditions. But
labeling these compass mechanisms as innate, even if they played no
role in higher order spatial cognition, would be an
oversimplification. Young birds must experience the sun’s arc across
the sky if they are to use it as a compass cue (Wiltschko and
Wiltschko, 1981). Even seeing the movement of the sun during only
one part of the day, for example the afternoon, enables young birds
to make meaningful inferences about the sun’s position at unfamiliar
times of day, in this case the morning (Budzynski et al., 2000).
Birds must continually adjust to the changing solar ephemeris due to
the shortening and lengthening of the day, a challenge compounded in
migrants because of their geographical displacements.
For
nocturnal migrants that use the stars to orient, similar experience
is required. Failure to see the night sky during their first summer
renders young birds unable to use the stars to guide their first
migration. However, even experience with a night sky rotating around
a false axis, like a planetarium sky rotating around Betelgeuse in
Orion, or a completely artificial rotating night sky is sufficient
to enable young birds to adopt the point of rotation as a migratory
reference. In the northern hemisphere, young experimental birds
during their first migration will orient away from the point of
rotation, or “south”, thus displaying meaningful migratory behavior
(Emlen, 1970).
The
type of deprivation experiment that easily identifies a crucial role
for experience in shaping how birds use the sun and stars as a
compass has not been carried out with respect to the earth’s
magnetic field. However, geomagnetic orientation is responsive to
experience, and this is most apparent when conflicting information
about the direction of migration is provided by the different
compass mechanisms.
Compass mechanisms: interactions among the different cues. Some
have described the orientation mechanisms of birds as “redundant”.
However, the term redundant, suggesting that the different sources
of compass information provide identical information, is clearly
inappropriate. There is nothing redundant about the earth’s magnetic
field when the sun or stars are obscured by clouds. Similarly, there
is nothing redundant about the sun or stars for birds near the
magnetic equator where the inclination of the earth’s magnetic field
would render geomagnetic orientation ambiguous. Multiple sources of
compass information are clearly adaptive. But multiple sources also
raise the question of whether orientation mechanisms are organized
hierarchically; is one source of information preferentially used
over the others, and might orientation to one cue be calibrated
against another?
The
answer to this question is not straightforward. For young birds
learning about environmental orientation cues during their first
summer, both North American and European species seem to
preferentially rely on celestial cues, in particular the sun and
patterns of skylight polarization, as a geographic reference to
define north. Young birds will in fact use celestial cues to
determine their migratory orientation with respect to the ambient
magnetic field (Bingman, 1983). The use of celestial cues to
calibrate orientation to the earth’s magnetic field is adaptive
because whereas the point of celestial rotation provides a
temporally and spatially stable reference to define geographic
compass directions, variation in the earth’s magnetic field in space
and time render it less reliable.
In
adult, experienced migrants the relationship between geomagnetic and
celestial orientation mechanisms depends on geographic location. In
Europe, magnetic field information is preferentially used to
calibrate orientation to celestial cues indicating an ontogenetic
shift in the hierarchy among the orientation mechanisms (Wiltschko
and Wiltschko, 1975). By contrast, in North America, at least at
more northern latitudes, celestial information continues to be
preferentially used to calibrate orientation to the ambient magnetic
field (Able and Able, 1990; Cochran et al., 2004). These findings
raise the question of why North American and European experienced
migrants should behave differently? A likely answer is related to
the relative stability of sun and geomagnetic information as birds
migrate in time and space (Bingman et al., 2003). As a bird migrates
south in North America, changes in the angular distance between
geomagnetic north and geographic north (declination) and changes in
the compass direction of the setting sun are similar. There would be
no advantage to shift away from the developmental pattern of
preferentially relying on celestial cues. By contrast, as a bird
migrates south in Europe, the angular distance between geomagnetic
north and geographic north remains essentially constant while the
direction of the setting sun changes. Therefore, for European
migrants, it would be adaptive to adopt the earth’s magnetic as the
preferential orientation cue once migration begins because of its
stability as a directional reference.
Compass mechanisms
enable birds to define directions in space to guide oriented
movement. However, a compass does not inform an organism of where
it is in space. That birds have a map sense of where, in addition to
a sense of direction, is readily attested to by the remarkable
ability of birds to return to the same breeding and wintering sites
year after year, and their ability to do so even after dramatic
experimental displacements; the most notable example of which is the
homing ability of pigeons. However, not all goal navigation
necessarily requires a map sense of where.
Getting there without knowing where. Many typically diurnal
songbirds will carry out their first migration at night alone in the
absence of any stable social network. Yet the vast majority of these
birds will reach their species typical over-wintering area often
thousands of kms away. It is difficult to imagine that such birds
have acquired map-like knowledge of their migratory route in the
absence of any previous experience, so how do they succeed? The
answer is a remarkable example of genetic programming (Berthold,
2003). Although the development of celestial sun and star compass
mechanisms requires experience, the initial orientation angle a bird
makes with respect to those cues seems to be innate. Once a bird is
able to define directional space using the sun, stars or earth’s
magnetic field, how they orient on their first migration, although
amenable to change, is innately represented in the nervous system.
This innate directional preference can get a naïve migrant moving at
least in the direction of its population specific over-wintering
site. In fact, the genetic programming can be so sophisticated as to
include appropriate changes in direction, for example, when some
European species shift their orientation from southwest to south as
they approach Africa (Wiltschko and Gwinner, 1974).
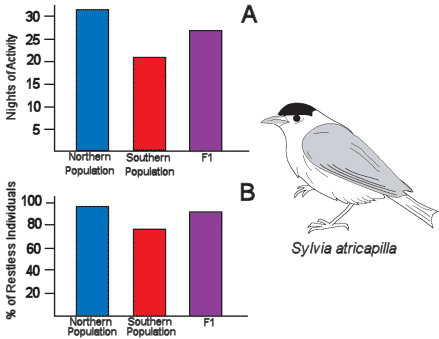 |
Figure 4:
Hand-raised black caps from a northern population
that naturally migrate farther (blue) display more
nights with nocturnal migratory activity (A) and a
greater percentage of migratory active individuals
(B) than black caps from a more southern population
(red). Crosses between northern and southern
population individuals produce F1 birds (purple)
that display intermediate levels of migratory
behavior.
|
But what
about distance, how does a naïve migrant know how far it should fly?
The solution to this challenge seems to be time (Figure 4). The
genetic program that appears to guide a young bird’s first migration
includes how long it should be active migrating (Berthold and
Querner, 1981). This was elegantly demonstrated by studying
different populations of European black caps (Sylvia atricapilla).
Identically hand raised young black caps from a long-distance
migratory northern population and a short-distance southerly
population were tested in cages for the amount of nocturnal activity
displayed during their first fall migration. Young birds from the
northern population displayed substantially more migratory activity
for a longer period of time during the fall compared to the southern
population. Interesting from a genetic perspective, crosses of the
northern and southern populations produced young that displayed
intermediate levels of migratory activity. The genetic program that
guides a young bird’s first migration seems to control distance by
controlling the amount of time a bird engages in migration.
In
summary, a young bird on its first migration succeeds in navigating
to its population specific over-wintering site without a map sense
of where. A genetic program that defines which direction and how
long to fly seems sufficient to get them close, and in the
literature this type of navigation is often referred to as “vector
navigation”.
Getting there and knowing where.As programmed as a young bird’s
first migration may be, experience provides them with opportunities
for a far richer representation of space that enables a map-like
sense of almost global proportions. This map sense can be used by
birds to navigate to specific goal locations following displacements
to unfamiliar places sometimes thousands of kms away. Layson
albatrosses (Phoebastria immutabilis),
white-crowned sparrows (Zonotrichia leucophrys), European
starlings (Sturnus vulgaris) and routinely homing pigeons (Columba
livia) are examples of species that have been used in
displacement experiments, successfully demonstrating ability to goal
navigate over unfamiliar terrain.
For a bird to have
a map-like representation of space, it needs to take advantage of
some spatial variation in the quality of environmental stimuli. For
a map of a familiar (experienced) environment, this variation may be
the spatial relationship among landmarks; such landmarks would be
typically visual (Biro et al., 2005; Gagliardo et al., 2001; Lipp et
al., 2004) but potentially of other sensory modalities as well. In
fact, the spatial relationship among the familiar landmarks and goal
locations is likely represented in a directional framework defined
by the sun or some other compass mechanism described above (Bingman
and Jones, 1994). An important point is that a bird would not be
able to extrapolate a map of familiar landmarks beyond the range of
sensory contact with the landmarks. But the map sense of birds seems
to extend well beyond the boundaries of the sensory range of their
experienced space.
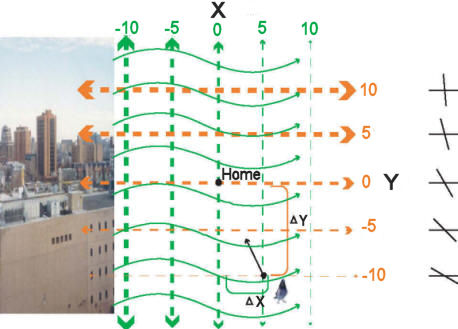 |
Figure 5:
Conceptualization of Wallraff’s gradient model of a
navigational map. In this hypothetical example,
variation in geomagnetic field inclination (far
right black lines) increases to the north (Y axis,
orange dashes of increasing thickness bracketed by
arrows). By contrast, a source of atmospheric odors
(city to the left) creates an odor gradient that
decreases to the east (X axis, green dashes of
decreasing thickness bracketed by arrows). A homing
pigeon transported to a location southeast of home
would measure its relative displacement by
determining the difference between the local
atmospheric odor intensity and geomagnetic field
inclination with the home values (relative values
ranging from +10 to -10). Once the direction of
displacement is determined, a homeward vector, or at
least direction, can be computed.
|
The
challenge of a map that extends beyond the range of experienced
space is that when a bird is displaced beyond the boundaries of
familiarity it must infer its location relative to a goal location.
As conceptualized by Wallraff (1974), a bird’s map of unfamiliar
space would be based on the qualities of two environmental stimuli
that vary predictably in space in a gradient like fashion
(Figure 5). The gradient axes of the two stimuli must also
intersect, not necessarily orthogonally, to create a bi-coordinate
grid-like system. Using the homing pigeon as an example, let’s
assume that with respect to the home loft the quality of stimulus x
increases to the north and decreases to the south, the quality of
stimulus y increases to the east and decreases to the west. A pigeon
learns the predictable properties of this variation during flights
over familiar areas. More importantly, what a pigeon learns has the
properties of an algorithm such that it can infer how the qualities
of the stimuli change beyond its area of familiarity. When a
pigeon is now displaced to the northwest beyond the range of
familiarity, it will detect an increase, compared to the home loft,
in the quality of stimulus x and infer its relative displacement
northward. It will also detect a decrease in the quality of stimulus
y and infer its relative displacement westward. The pigeon could
then essentially locate its position on the gradient map to compute
a vector or at least direction home to be read off one of its
compasses.
If
not the earth’s magnetic field, then what? Surprisingly, the answer
seems, at least in part, related to spatial variation in the
distribution of atmospheric odors (Wallraff, 2001). Numerous
experiments carried out in homing pigeons have demonstrated that
olfactory deprivation sabotages homing ability from distant,
unfamiliar locations while sparing homing from sites where familiar
landmarks can be used as an alternate source of navigational
information. More impressive, false olfactory information, in other
words releasing pigeons from one unfamiliar location while being
exposed to odors from another location, leads to predictable changes
in the direction flown by pigeons upon release. The orientation of
the “fooled” pigeons is consistent with them being released from the
site recognized by the odors and not their actual location.
Could variation in
the spatial distribution of atmospheric odors make up one or both of
the gradients in Wallraff’s model? Developmental studies have
demonstrated that even homing pigeons held in an outdoor aviary
without given the opportunity to fly can learn an olfactory
navigational map. Under these conditions, it is difficult to imagine
how a gradient map can be learned without a bird experiencing
quantitative differences in stimulus quality while actively moving
through space. Birds held in an outdoor aviary learn an olfactory
navigational map by associating different odor qualities with winds
from different directions. Rather than learn a gradient map, they
learn what has been described as a “mosaic map”, in which patches of
different atmospheric odor qualities are associated with different
compass directions (Papi et al., 1972). Note again that a compass
mechanism, like the sun compass, would be used to represent how
odors vary in space. When subsequently released from a distant,
unfamiliar location, a pigeon would sample the odor profile at the
release site, recall the wind direction associated with that odor
profile experienced at the loft and then, using its sun or magnetic
compass, fly off in a direction opposite from the associated wind
direction. Interesting, such a mechanism would render what are
ostensibly unfamiliar locations “familiar”. The odor profile of
unfamiliar sites would be “familiar” to the pigeons because of the
odor profile having been transported to the loft by winds. Odor
profile would take on the quality of a landmark that could be
experienced remotely because of wind.
So does such a
mosaic map of atmospheric odors completely solve the problem of
navigation after displacement to a distant, unfamiliar location?
Probably not. The primary obstacle is that successful navigation can
occur over hundreds of kilometers beyond a range conceivable for
wind borne odors to be reliably brought to one site like a pigeon
loft. How would a pigeon discriminate between an odor profile from
the north 50 kms away compared to one from the north 500 kms away?
It may well be that a mosaic map is operational over relatively
short distances (50-100 kms) and a gradient-like map is operational
over longer distances. But is there any evidence that pigeons can
learn two types of dissociable navigational maps? We will discuss
the role of the hippocampal formation in avian spatial behavior in
more detail below, but it is noteworthy that young homing pigeons
with hippocampal lesions are unable to learn an olfactory
navigational map when held in an outdoor aviary; a presumptive
mosaic map (Bingman et al., 1990). By contrast, young homing pigeons
with hippocampal lesions can learn an olfactory navigational map if
given the opportunity to fly freely from the loft under conditions
when the gradient quality in odor profile could be sampled as the
birds move through space (Ioalé et al., 2000). The different effects
of hippocampal lesions on navigational map learning under conditions
of varying experience are consistent with the two map idea. One
would be a hippocampal dependent mosaic map operational over
relatively short distances, the other a hippocampal independent
gradient map operational over much larger distances.
It must be admitted
that the proposal of an olfactory navigational map has not been
unanimously embraced by researchers in the field. A frequent
criticism has been the intuitive difficulty accepting that the
spatial variation in atmospheric odors is stable and predictable
enough in space and time to support a gradient or mosaic map of the
types described above. This criticism has now been successfully
answered by research actually measuring spatial variation in trace
atmospheric substances over distances homing pigeons routinely
return from. If one looks not at one substance but the relationship
among the concentrations of numerous substances, the spatial
variation of that relational quality is stable and
predictable enough to support a gradient map and explain how homing
pigeons can identify the direction home from hundreds of kms away (Wallraff,
2003).
We are comfortable
with the idea that homing pigeons can rely on atmospheric odors to
construct a navigational map, and that they do so in different
global regions with substantial differences in climate. There is
evidence that other species of birds can use a similar navigational
mechanism over relatively short distances (50-100 kms). But it seems
impossible to explain migrations of thousands of kms based on map of
atmospheric odors. What type of environmental stimulus could serve
as an element in a gradient map of this scale? Although not
necessarily satisfying given the general lack of empirical support,
and despite Wallraff’s admonishment (Wallraff, 1999), there is a
persistent temptation to think that at some point the answer will be
related to some variation(s) in the earth’s magnetic field. However,
one should be open to any theoretically possible solution as the
sensory and cognitive abilities of birds continue to offer
surprises.
Under
natural conditions, birds display an enormous range of spatial
behavior mechanisms including different compass mechanisms, vector
navigation, navigation by familiar landmarks, and mosaic and
gradient maps of atmospheric odors. But there is no reason to think
we have fully uncovered all the ways birds represent space or their
sensory basis. The different behavioral mechanisms would be
supported by different neural representational mechanisms, which
would to a lesser or greater extent be supported different brain
regions. To date, it is the hippocampal formation (HF) that has been
most extensively studied in the context of avian spatial behavior,
and not surprisingly, its importance appears restricted to only a
subset of the behavioral mechanisms described above. Although
playing some role in navigational map learning under conditions of
confinement in homing pigeons, the available data indicate that
prevailing role of HF in the spatial behavior of birds is in the
map-like representation of familiar landmarks used to guide goal
navigation over familiar terrain.
Lesion
and immediate early gene studies. The very first study examining
the effects of HF lesions on the homing behavior of experienced
pigeons was accompanied by the disappointment of beautiful homeward
orientation from a distant, unfamiliar location and the mystery that
the lesioned birds never showed up at the loft (Bingman et al.,
1984). How could one explain an intact navigational map but failed
homing? The hypothesis that was put forth was that as a pigeon
approaches its home loft it becomes increasingly reliant on familiar
landmarks to guide the final phases of the homing flight, and it is
navigation by familiar landmarks that engages HF. The importance of
HF for familiar landmark navigation has been demonstrated in
numerous field and laboratory studies, but we will only highlight
two to illustrate the complexity of this relationship.
Intact and HF lesioned homing
pigeons were trained from two familiar locations and then tested to
reveal the kind of landmark-based strategy they learned to return
from the familiar sites (Gagliardo et al., 1999). When tested, the
pigeons were rendered anosmic. Blocking the ability to smell would
eliminate the ability of the birds to rely on their olfactory
navigational map to return home, thus forcing them to rely
exclusively on their representation of familiar landmarks. They also
had their internal clocks phase-shifted. Conceptually, homing
pigeons could use familiar landmarks as an independent map and
guidance system, using the landmarks to guide their flight home by
serially locating their position in space and noting their movement
with respect to the landmarks. Alternatively, they could simply use
the landmarks at the familiar release site to recall the compass
direction flown from that site during training, and then use their
sun compass to take up the homeward bearing. Phase-shifting would
dissociate these two strategies. Navigating home by gauging movement
with respect to the familiar landmarks alone would not be influenced
by the phase-shift manipulation. By contrast, recalling the compass
direction home and then orienting by the sun would result in a shift
in orientation away from the homeward direction.
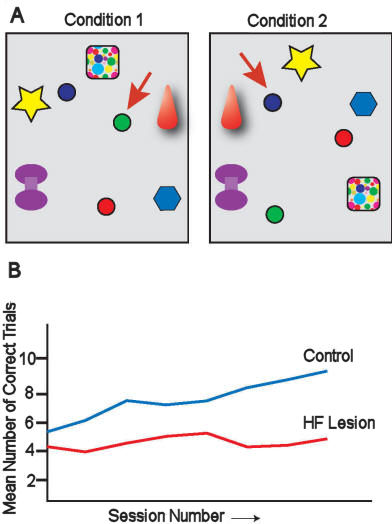 |
Figure 6:
A. Two five-landmark array environments that
differed in the spatial (topological) relationship
of the landmarks (e.g., the purple spool was
counter-clockwise of the star in one environment and
counter-clockwise of the red pyramid in the other).
The green bowl contained food in one environment
(arrow); the blue bowl in the other (arrow). The red
bowl never contained food. B. Control pigeons (blue
line) successfully learned to discriminate the two
landmark arrays to choose the correct food bowl. At
the end of training they were getting close to 90%
of all trials correct. Although the HF lesioned
birds (red line) learned to preferentially choose
the green and blue food bowls and not the red, they
never learned to associate the green and blue bowls
with the correct landmark array. |
The results of this study
demonstrate how subtle the differences can be in the navigational
strategies used by control and HF lesioned pigeons. Control pigeons
oriented in a direction approximating the true direction home, and
therefore, were for the most part uninfluenced by the phase-shift
manipulation. They used the unspecified array of familiar landmarks
in a map and guidance-like fashion. By contrast, the HF lesioned
pigeons displayed a shift in orientation away from the home
direction indicating that they relied on their sun compass once
determining their location relative to home, presumably by
recognizing landmarks at the training site and then recalling the
compass direction home flown during training. It is clear that the
map-like spatial memory representation learned by the control
pigeons was much richer in terms of spatial information available
and the potential for inferring route corrections in the event of
displacement. This ability requires recruitment of HF. Simply
learning to associate a compass direction with a cluster of familiar
landmarks, instructed by the olfactory navigational map available
during the training phase of the study, does not require an intact
HF.
It is
appealing to label the spatial learning of the control pigeons in
the previous study as reflecting map-like or spatial relational
learning; what has been called a cognitive map (O’Keefe and Nadel,
1978). However, under field conditions it is prohibitively difficult
to determine if landmarks are actually being used and how they are
represented (but see Guilford at al., 200x; Lipp et al, 2004); the
landmarks can’t be manipulated. In a companion study (Figure 6),
control and HF lesioned pigeons were trained to discriminate between
two landmark arrays, which varied with respect to the spatial
relationship among the landmarks, to determine which one of three
possible goal locations contained food (White et al., 2002). The
landmarks used in the two arrays were identical, just their spatial
relationship with respect to each other varied between the two
conditions. Control pigeons were successful in discriminating
between the landmark arrays. In striking contrast, the HF lesioned
pigeons gave no indication of learning that the spatial relationship
among the landmarks was different in the two conditions. This
laboratory study, together with the previously described field
study, offer compelling evidence that the avian HF is crucial for
successfully representing landmarks in a map-like, relational
manner; a map that can then be used to guide to navigation among
goal locations.
The usefulness of lesion techniques for the study of brain-behavior
relations is indisputable. However, it is desirable that conclusions
drawn from lesion studies be supported by less invasive experimental
procedures. One such procedure relies on the activation of so-called
immediate early genes that are thought to be often recruited when
some type of neuronal re-organization in support of learning occurs.
For both homing pigeons learning to navigate by familiar landmarks
(Shimizu et al., 2004) and a species of songbird remembering the
locations of cached seeds to be recovered later (Smulders and
DeVoogd, 2000), increased activation of an immediate early gene has
been observed in HF. Both the lesion and immediate early gene data
converge on the conclusion that the avian HF is critical for
map-like representations of space.
Unit
recording studies. The realization that the avian HF is crucial
when map-like representations are recruited to navigate and
recognize salient locations in space raises the challenging question
of how space may be represented at the level of the response
properties of HF neurons (units). As background to this question are
the well described “place cells” found in the rodent hippocampus
(O’Keefe and Nadel, 1978). Place cells are neurons that routinely
display large increases in activity when a laboratory rat is at a
restricted location in an experimental environment. The place cell
has shaped discussion of hippocampal function since its discovery
more than 30 years ago, and necessarily looms as a standard by which
HF unit response properties in other species are measured. However,
given the substantial differences in spatial ecology and
evolutionary history between rats and birds like homing pigeons, it
is likely that the spatial response properties of HF neurons would
differ between the two groups in some adaptive fashion.
|
Figure 7:
Video illustration of a homing pigeon, navigating an
analogue 8-arm radial maze (Hough and Bingman,
2004), with HF implanted electrodes connected to a
recording cable.
Click on Figure
to Start Video
|
In fact, recordings of HF neurons
carried out in freely moving homing pigeons navigating a laboratory
environment (Figure 7) have yet to reveal place cells so easily
encountered in rats. Rather, what have been found are neurons of two
types that are relevant to the challenges of navigating and
recognizing locations in space (Hough and Bingman, 2004; Siegel et
al., 2005; Siegel et al., In press). One type of neuron is
characterized by a tendency to display increased levels of activity
(action potential firing rate) when a pigeon is at or near a goal
location; a type of neuron we have referred to as a “location”
cell. Although perhaps superficially resembling place cells, these
pigeon HF location cells differ from rat place cells with respect to
a number of response property characteristics. The second type of
neuron is characterized by a tendency to display increased levels of
activity when a pigeon is moving through corridors that leads to and
from goal locations; what we have referred to as a “path”
cell. The types of response properties described are consistent with
the speculation that homing pigeon HF neurons participate in
relating the position of goal locations with the computation of
navigational trajectories that lead to those locations. But perhaps
the biggest surprise is that neurons with different response
properties tend to lateralize to the HF on different sides of the
brain.
A lateralized HF: Adaptation for navigating avian space?
The
functional lateralization of the vertebrate forebrain was once
thought to be a uniquely human characteristic. However, it has now
been clearly demonstrated that the avian forebrain is similarly
lateralized with the different hemispheres preferentially recruited
in the control and expression of different behavior (Güntürkün,
1997). This has been convincingly shown in the domain of spatial
behavior in a number of bird species such as chicks, homing pigeons
and songbirds. More recently, the asymmetrical contribution of the
HFs of the two forebrain hemispheres in guiding spatial behavior has
been revealed. In one lesion study carried out in homing pigeons
(Kahn and Bingman, 2004), birds were trained to locate a food goal
by relying on landmark cues locally distributed in the experimental
environment the birds could move through (Figure 8). They could also
rely on distal cues such as light fixtures and markings on the walls
and ceiling in the room where the experimental environment was
located. Pigeons with left and right HF lesions both learned the
task without difficulty. However, the spatial representation that
guided their behavior, as revealed by probe trials that set
information from the local landmarks in conflict with the distal
room cues, was notably different. Pigeons with left HF lesions
overwhelmingly relied on the distal room cues to locate the goal and
behaved as if the local landmarks did not exist. By contrast,
pigeons with right HF lesions used the distal room cues less and
were more reliant on the local landmarks to locate the goal. The
results suggest that the right HF may be more important for the
representation of goal locations reliant on global/distal properties
of an environment. It is interesting to note that pigeons with right
HF lesions can also use the sun compass to learn the location of a
goal or an olfactory navigational map; both conceptually
navigational abilities are impaired in pigeons with left HF lesions.
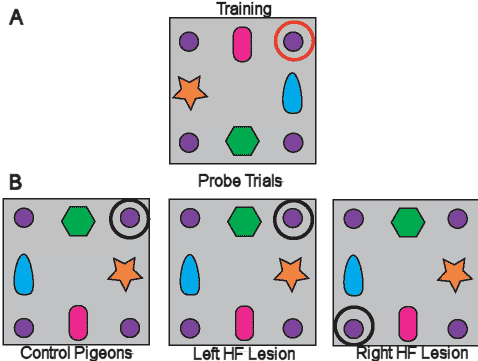 |
Figure 8:
In a lesion study carried out in homing
pigeons (Kahn and Bingman, 2004), birds were trained to
locate a food goal by relying on landmark cues locally
distributed in the experimental environment the birds could
move through. Figure A shows the goal location in training
while figure B shows the birds selection of the goal
location in probe trials.
|
The
different sensitivity of the right and left HF to different aspects
of space as revealed by the lesion studies is paralleled by unit
recording data (Siegel et al., In press). The occurrence of location
and path cells described above do not distribute symmetrically in
the HFs of the two hemispheres. Location cells are more likely to be
found in the right HF while path cells are almost exclusively found
in the left HF. The spatial response profile of neurons in the left
and right HF also differ in other respects, the most notable of
which is the greater temporal stability or reliability in the
spatial variation in firing rate of left HF cells. Neurons in the
left HF likely participate more in representing aspects of space
that are stable in time.
Reconciling the lesion and unit recording data.
Surveying the lesion and unit recording data reveals a complex
picture of HF function and its apparent defining characteristic of
lateralization. This lateralized quality is interesting because the
human HF is also lateralized while there is little indication of it
in the rat. When the dust settles, lateralization, and particularly
HF lateralization in the context of spatial behavior, may be a
defining adaptive feature of the avian HF organization that explains
in part the extraordinary ability of birds to navigate space (Bingman
et al., 2003). But what is really lateralized? As a working model,
we view the right HF as preferentially participating in the
representation of goal or “event” locations (location cells) defined
by global spatial features of the environment (lesion data). By
contrast, the left HF preferentially participates in navigating the
environment and computing trajectories among goal locations (path
cells) relying on map-like representations of landmarks learned
with the aid of directional cues like the sun compass. However, it
must be emphasized that the proposed functional asymmetry is in some
sense an experimental artifact. In intact pigeons, the two HFs work
cooperatively and collectively in supporting behavior; goal
navigation requires an ability to determine a path trajectory or
route as well as recognize the location of a goal once close. A very
large hippocampal commissure offers testimony that the two HFs
function as an integrated unit in the control of spatial behavior.
Indeed, in the field navigating by familiar landmarks as described
previously is disrupted by either left or right HF lesions (Gagliardo
et al., 2002). Neurons in the left and right HF may be
preferentially sensitive to different aspects of space, but both are
required to support the challenge of navigating by a map-like
representation of familiar landmarks.
Traditionally, the study of comparative psychology has relied on
controlled experimental settings in an intellectual setting shaped
by learning theory. Although undeniably successful as a science,
this research may have necessarily diminished the detection of
species differences as subjects were tested in laboratory
environments that often failed to promote the expression of species
typical behavior and the cognitive mechanisms that support them. The
research described in this chapter is inspired by a complementary
approach to comparative psychology that draws on the lessons of
ethology. It can be taken as axiomatic that during the course of
evolution species ecology and natural history have substantially
shaped the relationship among brain organization, behavior and the
underlying cognitive processes that support behavior. The unique
suite of spatial behavior mechanisms that birds rely on to navigate
space, from a magnetic compass and vector navigation that require
little experience to become operational to open-ended, HF mediated
familiar landmark navigation, can all be viewed as adaptive
responses to the challenges of their spatial ecology. From this
perspective it is easy to understand why the homologous HF of rats
and homing pigeons can differ in the qualities of space represented.
More subtle HF differences can be expected even among different
species of birds or any taxonomic group. In our view, a growth area
in comparative psychology is a revitalized interest in an
experimental philosophy that encourages the expression of species
typical behavior accompanied by research into supporting neural
mechanisms. The comparative study of spatial cognition is an example
of how successful this approach can be.
Able KP (1982) Skylight polarization patterns at dusk influence the
migratory orientation of birds. Nature 299, 550-551.
Able KP, Able MA (1990) Calibration of the magnetic compass by a
migratory bird by celestial rotation. Nature 347, 378-380.
Berthold P (2003) Genetic basis and evolutionary aspects of bird
migration. Advances in the Study of Behavior. 33, 175-229.
Berthold P, Querner U (1981) Genetic basis of migratory behavior in
European warblers. Science 212, 77-79.
Bingman, VP (1983) Magnetic field
orientation of migratory Savannah sparrows with different first
summer experience. Behaviour 87, 43-53
Bingman, VP, T-J Jones (1994) Sun compass based spatial learning
impaired in homing pigeons with hippocampal lesions. Journal of
Neuroscience 14, 6687-6694
Bingman VP,
Budzynski CA, Voggenhuber A (2003) Migratory systems as adaptive
responses to spatial and temporal variability in orientation
stimuli. In: Avian Migration.
(Berthold P, Gwinner E, Sonnenschein E. eds.) pp 457-469: Springer-Verlag,
Heidelberg-New York.
Bingman VP, Ioalé P, Casini G,
Bagnoli P (1990) The avian hippocampus: Evidence for a role in the
development of the homing pigeon navigational map. Behavioral
Neuroscience 104, 906-911.
Bingman VP, Bagnoli P, Ioalé P,
Casini G (1984) Homing behavior of pigeons after telencephalic
ablations. Brain, Behavior and Evolution 24, 94-106.
Bingman VP, Hough II, GE, Kahn MC,
Siegel JJ (2003) The homing pigeon hippocampus and space: In search
of adaptive specialization. Brain, Behavior and Evolution 62,
117-127.
Biro D, Meade J, Guilford T (2004) Familiar route loyalty implies
visual pilotage in the homing pigeon. Proceedings National Academy
Sciences, USA 101, 17440-17443.
Budzynski CA, Dyer FC, Bingman VP (2000) Partial experience with the arc of
the sun is sufficient for all day sun compass orientation in young
homing pigeons, Columbia livia. Journal of Experimental
Biology 203, 2341 - 2348.
Cochran WW, Mouritsen H, Wikelski M (2004) Migrating songbirds
recalibrate their magnetic compass daily from twilight cues. Science
304, 405-408.
Emlen ST (1967) Migratory orientation in the Indigo Bunting,
Passerina cyanea. Part II. Mechanisms of celestial orientation.
Auk 84, 463-489.
Emlen ST (1970) Celestial rotation:
its importance in the development of migratory orientation. Science
170, 1198-1201.
Fischer J, Munro U, Phillips JB (2003)
Magnetic navigation by an avian migrant? In: Avian Migration.
(Berthold P, Gwinner E, Sonnenschein E. eds.) pp 423-432: Springer-Verlag,
Heidelberg-New York.
Gagliardo A, Ioalé P, Bingman VP
(1999) Homing in pigeons: The role of the hippocampal formation in
the representation of landmarks used for navigation. Journal of
Neuroscience 19, 311-315.
Gagliardo A, Odetti F, Ioalé P (2001) Relevance of visual cues for
orientation at familiar sites by homing pigeons: an experiment in a
circular arena. Proceedings Royal Society London, B 268, 2065-2070.
Gagliardo A, Odetti F, Ioalé P,
Bingman VP, Tuttle S, Vallortigara G (2002) Bilateral participation
of the hippocampus in familiar landmark navigation by homing
pigeons. Behavioural Brain Research 136, 201-209.
Güntürkün O (1997) Avian visual lateralization: A review.
NeuroReport, 8(6), 3-11.
Hoffman K (1954) Versuche zu der im
Richtungsfinden der Vögel enthaltenen Zeitschätzung. Zeitschrift
für Tierpsychologie 11, 453 - 475.
Hough II GE,
Bingman VP (2004) Spatial response properties of homing pigeon
hippocampal neurons: Correlations with goal locations, movement
between goals, and environmental context in a radial-arm arena.
Journal of Comparative Physiology A 190, 1047-1062.
Ioalé P,
Gagliardo A, Bingman VP (2000).
Hippocampal
participation in navigational map learning in young homing pigeons
is dependent on training experience. European Journal of
Neuroscience 12, 742-750.
Kahn MC, Bingman VP (2004).
Lateralization of spatial learning in the avian hippocampal
formation. Behavioral Neuroscience 118, 333-344.
Kramer G (1952) Experiments on bird
orientation. Ibis 94, 265 - 285.
Kramer G (1959) Recent experiment on bird
orientation. Ibis 101, 399 - 416.
Lipp H-P, Vyssotski AL, Wolfer DP,
Renaudineau S, Savini M, Tröster G, Dell’Omo G (2004) Pigeon homing
along highways and exits. Current Biology 14, 1239-1249.
Moore FR (1987) Sunset and the
orientation behavior of migratory birds. Biological. Reviews 62,
65-86.
Mouritsen H, Frost BJ (2002 Virtual migration in tethered flying
monarch butterflies reveals their orientation mechanisms.
Proceedings National Academy Sciences, USA 99, 10162-10166.
O’Keefe J (1996) The spatial prepositions in English, vector
grammar, and the cognitive map theory. In: Language and Space (Bloom
L, Peterson MA, Nadel L, Garrett MF eds) pp. 277-316. MIT Press,
Cambridge, MA.
O’Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Oxford
University Press, Oxford.
Papi F, Fiore L, Fiaschi V, Benvenuti S, Baldaccini NE (1972)
Olfaction and homing in pigeons. Monitore Zoologico Italiano (N.S.)
6, 85-95.
Schmidt-Koenig K (1958) Experimentelle
Einflussnahme auf die 24 Stunden-Periodik bei Brieftauben und deren
Auswirkungen unter besonderer Berücksichtigung des
Heimfindevermögens. Zeitschrift für Tierpsychologie 15, 301-331.
Schmidt-Koenig K (1961). Die Sonne als Kompass im Heim-Orientierungssystem
der Brieftauben. Zeitschrift für Tierpsychologie 68, 221 - 224.
Shimizu T, Bowers AN, Budzynski C,
Kahn MC, Bingman VP (2004) What does a pigeon brain look like during
homing? Selective examination of ZENK expression in the
telencephalon of pigeons navigating home. Behavioral Neuroscience
118, 845-851.
Siegel JJ, Nitz D, Bingman VP (2005)
Spatial-specificity of single-units in the hippocampal formation of
freely moving homing pigeons. Hippocampus 15, 26-40.
Siegel JJ, Nitz D,
Bingman VP (In press) Lateralized functional components of
spatial cognition in the avian hippocampal formation: evidence from
single-unit recordings in freely moving homing pigeons. Hippocampus.
Smulders TV, DeVoogd TJ (2000)
Expression of immediate early genes in the hippocampal formation of
the black-capped chickadee (Poecile atricapillus) during a
food-hoarding task. Behavioural Brain Research 114, 39-49.
Wallraff
HG (1974) Das Navigationssystem der Vögel. R. Oldenburg, Munich.
Wallraff HG (1999) The magnetic map of
homing pigeons: an evergreen phantom. Journal of Theoretical Biology
197, 265-269.
Wallraff HG (2001) Navigation by
homing pigeons: updated perspective. Ecology Ethology Evolution 13,
1-48.
Wallraff HG (2003) Avian olfactory
navigation: its empirical foundation and conceptual state. Animal
Behaviour 67, 189-204.
White AR, Strasser
R, Bingman VP (2002) Hippocampus lesions impair landmark array
spatial learning in homing pigeons: A laboratory study. Neurobiology
of Learning and Memory 78, 65-78.
Wiltschko R, Wiltschko W (1981) The
development of sun compass orientation in young homing pigeons.
Behavioral Ecology and Sociobiology 9, 135 - 141.
Wiltschko W, Gwinner E (1974) Evidence
for an innate magnetic compass in garden warblers.
Naturwissenschaften 61, 406.
Wiltschko W, Wiltschko R (1972)
Magnetic compass of European robins. Science 176, 62-64.
Wiltschko W, Wiltschko R (1975) The
interaction of stars and magnetic field in the orientation systems
of night migrating birds. II. Spring experiments with European
robins (Erithacus rubecula) Zeitschrift für Tierpsychologie
39, 265-282






