Geometry, features, and orientation in vertebrate
animals: A pictorial review
Ken Cheng and Nora S. Newcombe
Macquarie University & Temple University
Neurophysiology
Rats
The hippocampus is well known for its role in spatial navigation (O’Keefe & Nadel, 1978). It is beyond the scope of this chapter to review the literature on the hippocampus and space (review: Jeffery, 2003). But we present here some results concerning the encoding of geometric properties.
 |
|
Figure I-1: |
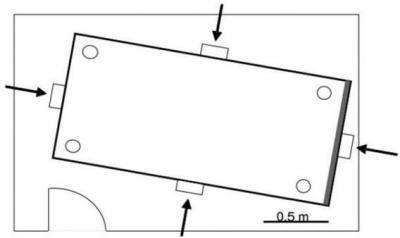
Hippocampal lesions in rats reduce rats’ ability to choose the geometrically correct corners of a rectangular pool (Pearce et al., 2004). The pool (Figure I-1) is surrounded by a circle of curtains, and would be filled with water during experimentation.
 |
| Figure I-2:
O’Keefe and Burgess (1996) apparatus. Thanks to Neil Burgess for this picture. |
O’Keefe and Burgess (1996) recorded from the rat’s hippocampus as the rat wandered in a rectangular space. The rat was oriented and had external cues to direction. It searched in the white rectangle, whose shape can be changed by shifting the walls appropriately. Hippocampal place cells (O’Keefe & Nadel, 1978) are typically found in the hippocampus proper. They fire preferentially when a rat is at a particular place in space.
 |
| Figure I-3: Adapted from Figure 1, O’Keefe & Burgess, 1996. Thanks to Neil Burgess for the picture. |
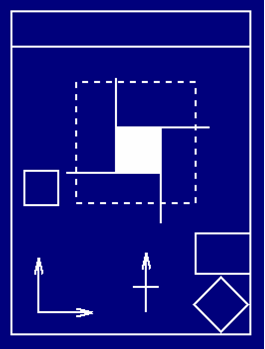
In this figure, the ‘training’ space was the vertical rectangle on the bottom left of each panel. The rat was then transferred to transformed spaces (the other diagrams) and the firing rate of each cell recorded. What the pattern of results from 28 cells show (see also Hartley, Burgess, Lever, Cacucci, & O’Keefe, 2000) is that these hippocampal cells are sensitive to both absolute distances from walls, and to relative distances, varying according to circumstance. Below in this section, we present the group’s model explaining this.
 |
| Figure I-4:
Adapted from Burgess et al. (2000, Figure 1). Thanks to Neil Burgess for the picture. |
The ‘performance’ of 35 recorded place cells are shown here. These firing rates are obtained independent of the direction that the rat is facing.
 |
| Figure I-5:
Taken from Lever et al. (2002, Figure 1). |
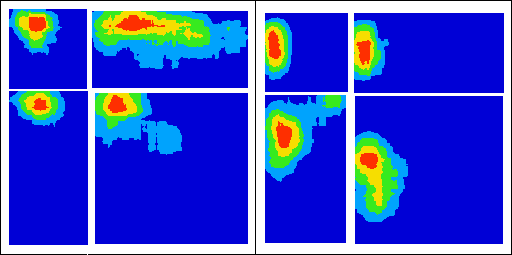
The shape of the ‘training’ space can be more radically transformed, and the hippocampal cells will still show systematic firing patterns (Lever, Wills, Cacucci, Burgess, & O’Keefe, 2002). For example, a square may be turned into a circle. Often, cells would fire at corresponding locations in the two spaces.
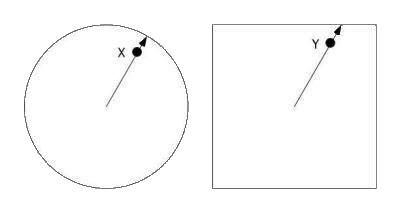 |
| Figure I-6: |
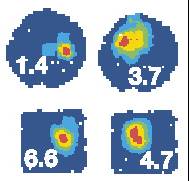
Correspondence is determined as follows. If X is the place of maximal firing in the training space (left), then Y, the place of maximal firing in the transfer space (right) lies in the same direction from the center, at the same proportion of distance from the center to the edge.
 |
| Figure I-7:
Taken from Figure 3 of Lever et al. (2002). |
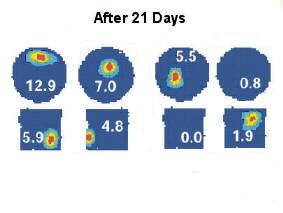
Over many days of repeated exposure to both spaces, however, the ‘behavior’ of the cell in the two spaces may change. After 21 days, a cell might respond in one space but not the other (right), or might respond at different, non-corresponding places in the two spaces (left).
 |
| Figure I-8: Adapted from Hartley et al. (2000, Figure 1). Thanks to Neil Burgess for the picture. |
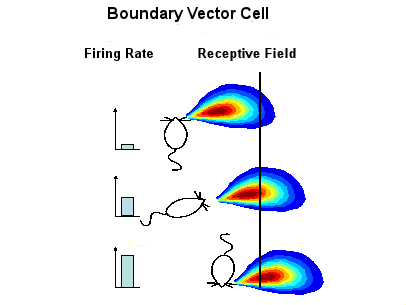
To model the performance of place cells, the existence of boundary vector cells are posited (Burgess et al., 2000; Hartley et al., 2000). These cells, which have not to date been found, respond when the rat is at a particular distance and direction from a surface (the black line), independent of the direction that the rat is facing. The boundary vector cells are upstream from the place cells, and a conjunction of boundary vector cells determine the performance of a place cell.
This discussion of place cells in the rat hippocampus suggests that these cells perhaps play a role in encoding local geometry, vectors to surfaces, and not the global orientations that determine which direction is which.
 |
| Figure I-9: Adapted from Golob et al. (2001), Figures 1 and 2 |
Other cells in the hippocampal complex, called head direction cells, are sensitive to the direction that the rat’s head is facing, irrespective of the place that the rat is currently at (review: Dudchenko, 2003). These cells are typically found outside the hippocampus proper, in diverse areas ranging from the lateral dorsal thalamus to the dorsal tegmental nucleus. As these cells appear to encode direction, we may wonder whether their performance has something to do with encoding geometry and with rotational errors.
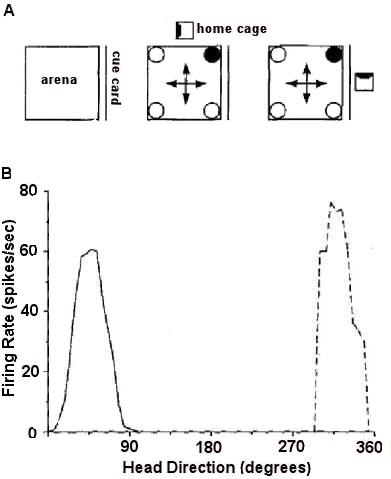
Golob, Stackman, Wong, and Taube (2001) probed this question. Head direction cells were recorded while a thirsty rat was in a square arena (Figure I-9A). The rat was not disoriented, but circular curtains surrounded the arena. Its home cage might change locations on different trials, as illustrated in Figure I-9A. A cue card on one wall provided directional cues. The rat had the behavioral task of finding one constant corner at which water was available; this was thus a reference memory task. The performance of a head direction cell was generally consistent, but on occasional trials, its preferred direction might shift by some multiple of 90o, as the dashed curve in Figure I-9B shows.
The question that arises is whether these shifts in the performance of head direction cells predict behavioral errors on the part of the rats. Across a number of experiments, the answer was a clear no. Golob et al. found no significant relations between the preferred direction of head direction cells and behavior.
Birds
 |
| Figure I-10:
Thanks to Giorgio Vallortigara for the pictures on chicks in this section. |
Many birds show many hemispheric specializations for behavior. Visual input to the bird brain is highly lateralized. By covering one eye, as in this picture, information is effectively restricted to half the brain. With the left eye covered, this chick is a left-brained animal visually.
 |
| Figure I-11: Procedure and results from Vallortigara, Pagni, and Sovrano (2004) |
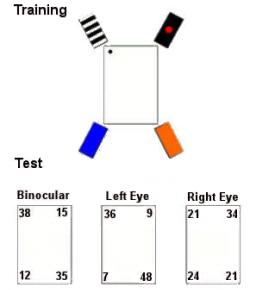
Vallortigara, Pagni, and Sovrano (2004) used eye covering to test the role of the hemispheres in encoding geometric and featural cues. In this experiment, chicks were trained with geometric and featural cues, but tested for their use of geometric information. Binocular and left-eyed (right-brained) chicks did fine, while right-eyed (left-brained) chicks failed.
 |
| Figure I-12: |
This experiment tested the use of featural cues. In the tests, geometric cues were removed by making the space square. Both left-eyed and right-eyed chicks did fine, indicating that both hemispheres encode featural cues.
 |
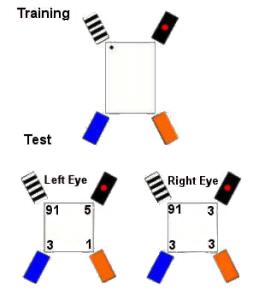
| Figure I-13: |
In this experiment, both the training and test situations had only geometric cues. Left-eyed (right-brained) chicks again did fine. Right-eyed (left-brained) chicks were marginal (did not quite beat chance statistically, at p = 0.06).
The study shows that the left hemisphere encodes only features, while the right hemisphere encodes both features and geometry. Tommasi, Gagliardo, Andrew, and Vallortigara (2003) used lesions of the right or left hippocampus of chicks in the relocation task. They came to similar conclusions. The left hippocampus encodes only featural information, while the right hippocampus encodes geometric and featural information.
 |
| Figure I-14:
Adapted from Vargas et al. (2004, Figure
1).
|
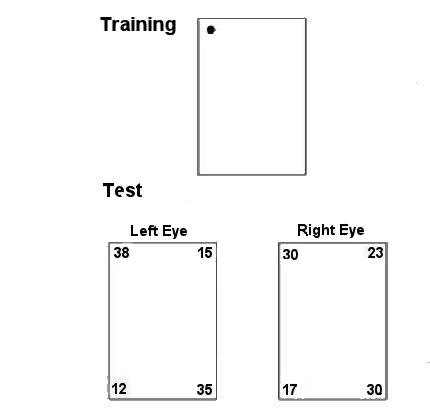
The role of the hippocampus in geometric encoding has also been studied in homing pigeons (Vargas et al., 2004). Normal pigeons and birds with their hippocampus lesioned (bilaterally) were trained in a space containing both featural and geometric cues. Both learned the task, but interestingly, birds without hippocampus reached criterion faster. It seems that learning geometry (in birds with hippocampus) is obligatory, even when it does not help. Even though geometric information is ambiguous and may cause rotational errors, it is nevertheless learned. This point is also clear from the studies on cue competition.
 |
| Figure I-15:
Adapted from Vargas et al. (2004, Figure 4). |
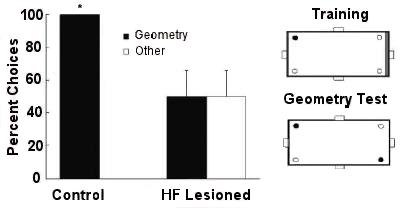
When featural cues (a red wall) were removed on a geometry test, control birds used the geometric cues, whereas birds with their hippocampal formation lesioned failed.
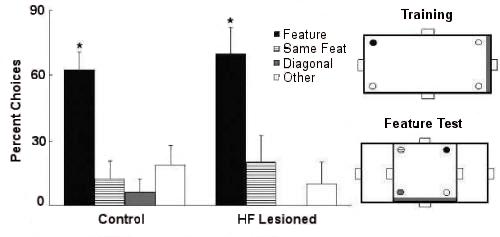 |
| Figure I-16: Adapted from Vargas et al. (2004, Figure 5). |
When geometric cues were removed by making the search space square on a feature test, both control and lesioned birds succeeded.
 |
| Figure I-17:
Adapted from Vargas et al. (2004, Figure 6). |
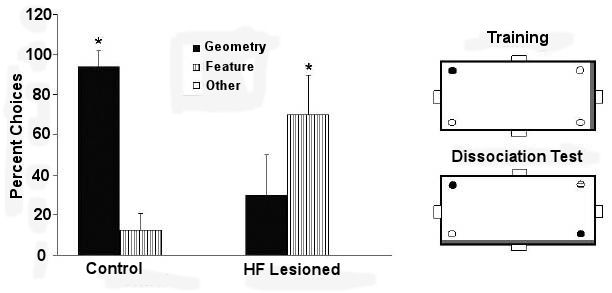
When geometric cues were put in conflict with featural cues, by making a long wall rather than a short wall red, control birds went with the geometry whereas lesioned birds went with the features.
Humans
Parahippocampal Place Area?
It has been proposed that the geometric layout of the environment is constructed in a specific area of the human brain, namely the posterior tip of the parahippocampal gyrus and adjacent regions of the fusiform gyrus (Epstein and Kanwisher, 1998; Epstein, DeYoe, Press, Rosen, & Kanwisher, 2001; Epstein, Graham, & Downing, 2003; Epstein, Harris, Stanley, & Kanwisher, 1999). This area has been termed the Parahippocampal Place Area (PPA).
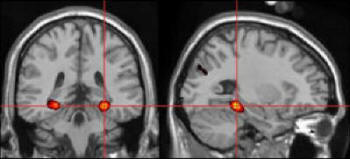 |
| Figure I-18:
This and the next few images come from Russell Epstein. |
Human Imaging Evidence
Perception of scenes led to differential activation in the PPA, relative to perception of faces, objects, or houses (Epstein & Kanwisher, 1998), even though the subjects were not required to perform any tasks.
 |
>
|
 |
| Figure I-19: Scenes used by Epstein & Kanwisher (1998) |
||
More Evidence on PPA and Geometry
Coherent geometric structure seemed vital to PPA activation because fractured and rearranged versions of the bare rooms did not elicit a response.
Further, activity in the PPA is not further increased when people feel as if they are moving in a scene, suggesting that it is more involved in geometric analysis than in planning routes or monitoring locomotion (Epstein et al., 1999).
PPA Seems Viewpoint Invariant
Imaging of PPA activity while people looked at scenes such as those in the next figure showed that a change in viewpoint (middle row) led to as much activity as a complete change in place (at bottom) with both elevated relative to no-change control (at top).
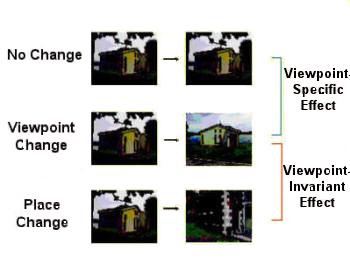 |
| Figure I-20: |
Doubts About the PPA as Possible Substrate for Geometric Module
There are alternative interpretations of the role of human parahippocampal cortex (Maguire, Burgess, et al. , 1998; Maguire, Frith, et al., 1998; see also Maguire, Burgess, & O’Keefe, 1999; O’Keefe et al., 1999). They have imaged humans performing a variety of tasks such as navigating around a virtual town (see next figure)
This group argues that the parahippocampal area is especially involved in locating objects in allocentric space. In fact, Burgess and O’Keefe (2003) suggest that the human parahippocampus is better termed a Spatial Scene Area than a Place Area.
 |
| Figure I-21: Virtual Reality paradigm used by Burgess and colleagues. Picture courtesy of Neil Burgess. |
Place Cells in the Hippocampus?
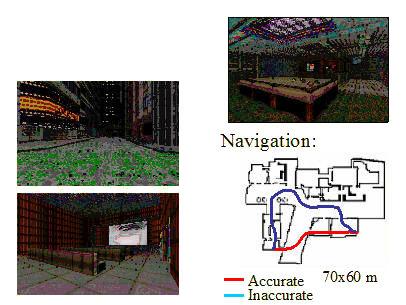
Ekstrom et al. (2003) conducted the first single-cell investigation of humans--7 patients with pharmacologically intractable epilepsy who were being observed with intra-cranial electrodes participated as taxi drivers in a VR navigation game (Figure I-22, courtesy of Michael Kahana). They picked up passengers and took them to one of nine distinctive buildings arranged in a grid.
 |
| Figure I-22: Example stimulus (left) and stimulus layout (right) in Ekstrom et al. (2003) |
Cells were categorized as place cells if they responded when the patient was in a particular location in the grid, independent of what view was on the screen or what goal was being sought. Similarly cells could be categorized as view cells or as goal cells if they responded to particular views or goals but were not affected by the other two types of information or by interactions. Place cells predominated in the hippocampus (as shown Figure I-23, courtesy of Michael Kahana) and view cells in the parahippocampus.
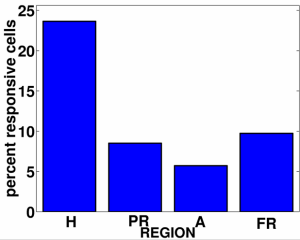 |
| Figure I-23: Ekstrom et al. data |
|