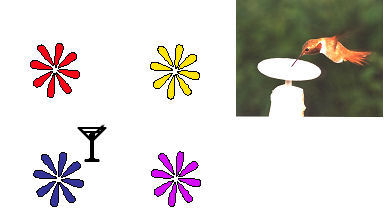Variation in spatial cognitive ability has been both a cause for
concern and enthusiastically observed in almost equal measure. The
cause for concern comes, typically, from studies in which the aim is
to examine performance on a task such that, as nearly as possible,
only a particular cognitive ability is assessed. External factors
such as time of day, time of year, temperature, light intensity and
many more are controlled by the experimenter so as to have no, or
negligible, effect on performance. Intrinsic factors such as
motivation (e.g., appetite and thus willingness to work for food
rewards) are more difficult to control but these too are manipulated
so as to reduce variation both within and among subjects. Previous
experience can be essentially removed as a source of variation by
training animals to a pre-test criterion level. If they do not
reach this level, they are excluded from experimental testing. All
of this control by the experimental psychologist leads to a
significant reduction in the effects by extraneous factors on task
performance. To an evolutionary biologist, on the other hand,
variation in a trait is of as much interest as is the level of the
trait itself, not least because variation is what natural selection
gets to act on. Seeking out possible sources of variation, for
example, the need by one animal for more, or greater dependence on,
spatial information than another, also provides an a priori basis
for formulating adaptive hypotheses. Having some understanding of
the source of variation in need for spatial information means that
it is possible to explore whether or not natural selection has managed
to mould animal cognition with the same precision as it has shaped
sensory processing.
This difference in angle of approach to variation in spatial
cognition is proving to be a fruitful one even if sometimes beset
with misunderstandings (e.g., Hampton, Healy, Shettleworth, & Kamil,
2002; Macphail & Bolhuis, 2001). Some of the fruits of this approach are epitomized in
the work carried out by Balda, Kamil and colleagues, described
elsewhere in this volume. As a result, we can see that there is real
biological variation in spatial learning and memory, and we are
beginning to see what kinds of variation exist, the degree to which
variation occurs and under what circumstances. Here, I will examine
a more diverse literature than that described by
Balda and Kamil, this
volume, which
has also taken an adaptationist stance when addressing questions of
spatial cognition, and attempt to address the value and pitfalls of
such an approach.
Just as evolution via natural selection has acted on
morphological structures (Figure 1) so it has on cognitive abilities
(see also Balda & Kamil, this volume).
However, one of the major problems in determining what solutions have
been arrived at is that cognition itself is not as readily observed
as is a morphological structure. It is a behavioural response
(or lack thereof) to a stimulus and can be considerably more plastic, and
its presence more difficult to confirm, than most morphological
traits. It can also be difficult to differentiate between
aspects of cognitive abilities that are essentially innate and those
that are acquired through experience.
A useful place to begin to deal with this problem of identifying a
cognitive ability, its extent and variation, is with an
understanding of the specific problems an animal faces. In
spatial cognition this has lead to a focus on those animals that
appear to face considerable, even excessive, demands on their
ability to learn about and to remember locations.
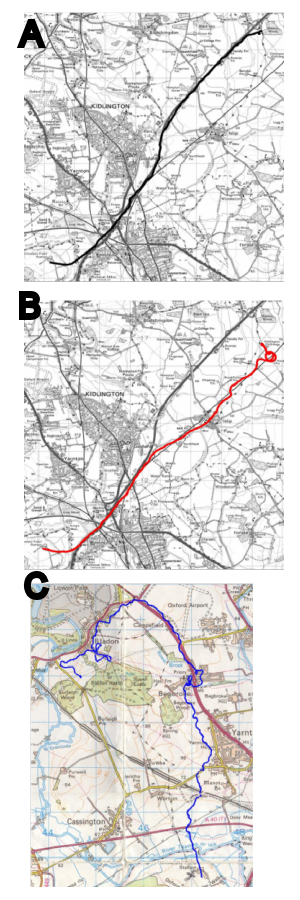
An example of an animal that faces a major spatial problem, which
has been more thoroughly investigated than others, is the homing
pigeon, a strain of Columba livia, which has been selected
for its ability to home to a loft over many miles. Most of the
work on pigeon navigation over both familiar and unfamiliar terrain
has focused on their ability to do this using magnetic and olfactory
cues, largely due to the finding in the 1970's that pigeons wearing
frosted contact lenses managed to reach the vicinity of their home
loft (Schmidt-Koenig & Walcott, 1978).
Bingman et al. (this
volume) present the current status of the work on homing in pigeons
from which it is clear that visual landmarks play a crucial role in
successful homing, although the scale over which those landmarks
operates means they are largely outside (as yet) experimental scope.
In addition to using olfactory and magnetic information, pigeons
utilize all manner of different visual landmarks, including those
humans have unwittingly provided (Biro, Meade, & Guilford, 2004;
Guilford, Roberts, Biro, & Rezek, 2004, Figure 2).
A
number of studies have demonstrated differences in cue use amongst
animals that are compatible with predictions based on the ecology of
the animals under comparison.
Food-storing birds, for example, which use
their memory for where they have hidden food to relocate it
successfully, prefer to use the spatial arrangement of visual cues
to do this (Sherry & Vaccarino, 1989). They also
preferentially use these kinds of cues when returning to sites
containing food previously seen but not removed (Brodbeck, 1994;
Clayton & Krebs, 1994). Nonstoring species, on the other hand,
tested in the same experiments, showed no particular preference for
which cues they used to return to sites containing food. This
difference in cue preference is consistent with the cue use
predicted for animals that differ in the degree to which they are
dependent on spatial information for efficient foraging or homing.
If one looks across the range of experiments carried out on cue
use in vertebrates, it becomes clear that many animals use the
arrangement of visual cues surrounding the goal, rather than visual
features of the goal itself, especially, but not exclusively, when
the goal itself is not particularly visually distinctive. This
has lead to a vigorous debate as to the roles of proximal or distal
cues, when it seems that examining the relative value of the
information provided by the cue may be more relevant.
For example, whether a cue is stable, more accurate or reliable
across time seems what is likely to be important
and useful to a navigating animal (Gibson & Shettleworth, 2003).
That cue use for solving apparently spatial problems may be context
dependent is further supported by data from a cue test in which
migratory and resident dark eyed juncos (Junco hyemalis
and J. h. carolinensis, respectively) were compared (Cristol
et al., 2003). Although the migratory subspecies outperformed
the resident subspecies in the duration over which they could
remember which was the correct feeder, both groups of animals showed
a marked preference for the spatial cues over the colour of the
feeder. These data are not consistent with the hypothesis that
cue preference is ecologically determined, at least, not in a
simple, across-context fashion
as in this situation, it might be predicted that the migrants would
be more dependent on spatial information than the residents.
It is also not clear why the outcome of this experiment was different from the
cue use found in comparisons
of migrant juncos
with chickadees (Brodbeck, 1994; Brodbeck & Shettleworth, 1995). In
a more recent, larger scale test of spatial memory, the two
subspecies did not differ in their ability to home from distances of
up to 40km (Keiser, Ziegenfus, & Cristol, 2005). This result shows that
demonstrating a clear relationship between ecological demands and
cognitive performance is far from straightforward.
The junco data from both labs also
shows that performance as shown under laboratory conditions may not
be representative of what animals do when tested in the wild.
One
of the few paradigms in which cue use has been tested repeatedly
in both laboratory and field conditions, is that of homing in
pigeons (see Bingman et al.,
and Phillips et al., this volume). However, most of the work has been directed at
determining whether directional cues such as the sun compass are
used or, whether information that the pigeons garner when flying can
help them, subsequently, to discriminate among photographs of a
variety of locations (Dawkins, Guilford, Braithwaite, & Krebs, 1996;
Wilkie, Willson, & Kardal, 1989).
It would be interesting to carry out experiments on pigeons similar
to those done with the juncos.
Aside from the homing pigeon literature, there have been few field
experiments on the use of visual cues by vertebrates to return to
rewarded sites. One exception is a series of tests done using rufous
hummingbirds. The choice of this species was based on both
logistical and biological features.
The first comprises such useful attributes as the birds coming to
feed every 10-15 minutes, strong territorial defence and
birds being readily trained to feed from artificial flowers. The biological rationale is based on the
territorial defence by the males of a number of flowers coupled with
the relatively small amount of time spent foraging and the fact that
the bird empties the flowers he visits. It is, therefore,
plausible that it might be efficient for these birds to remember, in
order to avoid, flowers they have recently visited (as they are now
empty).
It seems that territorial hummingbirds, like food-storing birds and
juncos, rely more heavily on spatial information than on visual cues
borne by flowers themselves when returning to rewarding flowers
(Healy & Hurly, 1998; Hurly & Healy, 1996; Miller, Tamm,
Sutherland, & Gass, 1985,
Figure 3). This finding appears to contrast with claims that the red
coloration of the Californian flora is due largely to the fact they
are, almost exclusively, pollinated by hummingbirds (Grant, 1966).
It is possible to reconcile the experimental findings with the
pollination hypothesis, if the birds in question are territorial in
the first instance and migratory in the second. Spatial memory
is only useful in familiar situations while using colour as a cue to
locate new sources of food is useful in unfamiliar places.
Thus, the same animal may pay attention to different cues depending
on the situation in which it finds itself. Territorial
hummingbirds can, and do, pay attention to the colour of flowers but
only under certain circumstances (Healy &
Hurly, 1998; Hurly & Healy, 1996).
One of the potential problems with basing adaptationist predictions concerning cue preference on the extent to
which species rely on spatial information, is that those species
will also tend to differ in a variety of other ways. It is
possible, for example, that an explanation for the difference in cue
use between black-capped chickadees and dark-eyed juncos may be
found in the placement of the feeders on the walls of the
experimental room. Although chickadees are familiar with feeding on
vertical substrates, this is uncommonly so for juncos. One of
the differences between the Brodbeck studies (Brodbeck, 1994;
Brodbeck & Shettleworth, 1995) and those by Cristol et
al. (2003) is that juncos were tested on the floor of the experimental room
in Cristol's tests. While it is not clear why this would
result in a change in cue use, it is at least possible that
experimental context is relevant. Even those experiments that
have examined cue use with much more closely related species that
appear to occupy very similar habitats and niches within them (e.g.,
Clayton & Krebs, 1994), face this problem. It is entirely
possible that there are other differences between these species that
cause them to pay more or less attention to visual cues (for
example), or aspects of those cues. If their visual processing
differed in some way, for example, or one species put more emphasis
on other sensory cues, then this may be the cause of the variation
in cue and not the apparent variation in dependence on spatial
information.
Given the importance of homing in the lives of homing
pigeons, it might be interesting to make a direct comparison of
spatial abilities between homing pigeons and laboratory strains of
pigeons, in addition to testing homing pigeons in experiments akin
to those used for investigating cognition in laboratory pigeons
(e.g., Dawkins et al., 1996). The relatedness, and hence marked
similarities, of the two groups might allow for a closer examination
of variation in spatial abilities due to ecological pressures or to
the impact of environmental variables than is usually possible
between animals from different species. Not only has there
been specific selection for navigational abilities in homing
pigeons, their hippocampus is larger than those of strains of
pigeons bred for apparently attractive morphological features, e.g.,
fantails and strassers (Rehkamper, Haase, & Frahm, 1988). This neural
difference might suggest that there are also differences in spatial
cognition between these groups.
Perhaps the largest body of work investigating which cues are used
to solve spatial problems is that comparing cue use between male and
female mammals. Although there is much debate about the cause
of the difference in cue use (for a review see Jones, Braithwaite, &
Healy, 2003),
it is a common finding that males use geometric cues (such as
distance and direction) while females typically use visual
landmarks. This finding is quite consistent across a range of
tasks requiring some kind of spatial ability for a correct or more
rapid solution (e.g., map drawing, Spencer & Weetman, 1981; giving
verbal directions, Dabbs, Chang, Strong, & Milun, 1998; moving around a virtual maze,
Sandstrom, Kaufman, & Huettel, 1998; real world navigation tasks,
MacFadden, Elias, & Saucier, 2003). Similarly, in maze testing, male and female
laboratory rats prefer to use these different cues (Kanit et al.,
1998, 2000; Williams, Barnett, & Meck, 1990). Little has
been done to investigate sex differences in cue use in other
vertebrates, although in one of the few bird comparisons, there was
no difference in cue preference between male and female great tits
(Hodgson & Healy, 2005). This lack of difference might have
been an outcome of the experimental design: birds were
required to visit a rewarded location multiple times before being
tested for cue preference. Although it is not clear whether
there would be a sex difference in these birds if preference was
tested after a single experience of the rewarded site (as has been
done in previous cue preference tests in birds), this finding emphasises the point made earlier that cue preference may be highly
responsive to experimental design (Figure 4).
|
|
 |
|
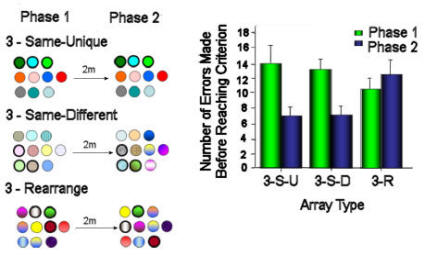
Figure 5. Use of spatial rather than visual cues by
hummingbirds in an array of flowers, three of which were
rewarded (marked by heavy black lines). Birds were trained
with arrays as seen in the left hand column. The arrays
were then moved 2m and birds trained with rewarded flowers in
the same or new positions, bearing the same or new colour
patterns. Birds did not appear to have learned, or to use,
the colour patterns of the flowers in Phase 1 when learning
which were the rewarded flowers in Phase 2. After Hurly &
Healy (2002).
|
|
|
 |
|
|
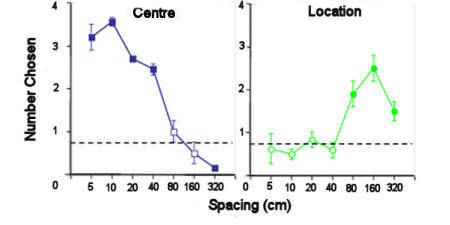
Figure 6. Rufous hummingbirds were trained to
feed from the central flower in an array of flowers and the
array was then moved such that the new centre was in the same
location as one of the outer flowers. When the flowers in
the array were 40cm apart or closer, the birds returned to the
flower in the centre. When they were further apart, the
birds returned to flowers in the original location. After
Healy & Hurly (1998).
|
A second way to make comparisons of spatial cognition
is to assess learning and memory, rather than cue preferences.
Ideally, one would have some understanding of the cue preferences
when comparing learning and memory, although examining factors such
as the ease with which animals reach a criterion before performance
is assessed may provide this to some extent.
Tests of cognitive ability followed the
cue preference tests in rufous hummingbirds. The question was then, what would one
predict these birds to use for ready remembrance of flowers if one
was to follow an adaptationist train of thought? Although the
hummingbirds prefer spatial (an arrangement of unspecified visual
cues) over beacon-like, visual cues does not mean the birds should
not learn and remember the visual information provided by flowers.
However, if the bird has to remember a flower for several hours, it
seems more likely that the flower's visual features are far more
likely to have altered than its spatial location. In fact, it
appears that even over the course of far shorter periods of time,
birds trained to find several neighbouring rewarding flowers in an
array failed to show any evidence of having learned the visual cues
of the flowers when trained on a similar array that had been moved
2m from the original location (see Figure 5, Hurly & Healy, 2002).
It made no difference to the birds' learning which were the rewarded
flowers in the new array, whether the flowers in this second array
were of the same or different colour patterns as those in the first
array, as long as the flowers were in the same relative positions.
Not until the shape of the test array, as well as its location, were
altered from the training situation did it become clear that the
birds had learned the colour patterns of the flowers in addition to
their positions in the array.
If the array is moved such that part of it overlays the
location of the original, the birds can be shown to encode which are
the rewarded flowers relative to the distance among the flowers:
flowers that are further than 40cm apart are encoded relative to
cues surrounding the array, while flowers that are closer together
than this are encoded relative to each other (see Figure 6, Healy &
Hurly, 1998). With regard to the adaptationist model, it is not
clear that these latter results would have been predicted, even with
greater knowledge of the animal's behaviour. However, it is
the case that without the original ecological rationale that
hummingbird foraging might be a fertile field for investigation into
spatial learning and memory, it is unlikely that findings such as
the scale of cue use would have come to light. Variation in
hummingbird foraging style (territory defence and traplining, where
animals travel a repeated route around widely spaced food sites) might
provide further insights into both the ways that animals use visual
and spatial information as well as offer the possibility that the
different foraging styles are accompanied by differences in
cognition. Traplining hummingbirds forage in a markedly
different way from that of territorial hummingbirds. For example,
they feed on flowers that decrease nectar production during the day,
a pattern that the birds mimic in their visitation rates whereas
territorial birds feed at a relatively constant rate throughout the
day (Garrison & Gass, 1999; Gass & Garrison, 1999). As a
result, traplining birds may be particularly good, for example, at
learning the sequence of places (Crystal & Shettleworth, 1994), or
may only encode food sites in a sequence (de Perera, 2004), rather
than in some more flexible way that would allow birds to access
sites at any time from any place (a.k.a. a cognitive map).
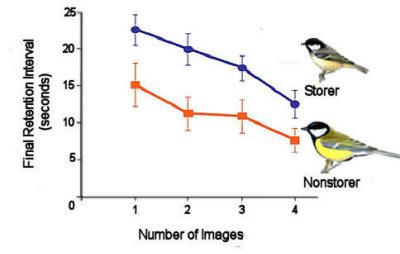 |
Figure 7. Coal tits (a food storing species) and
great tits (a nonstoring species) were trained and tested on a
spatial delayed non-matching-to-sample task presented on a touch
screen in which the number of samples, the duration of the
retention interval and the distance the choice images were apart
were all manipulated. The outcome was that coal tits were
better at remembering even a single location for longer than
were the great tits. After Biegler et al. (2001).
|
Another example in which cue preference was known, food storing,
provided an ecological rationale for the hypothesis that
scatter-storing (as opposed to larder storing) animals, with their
greater dependence on spatial memory for successful retrieval of
their stores, would outperform nonstorers on spatial memory tasks, a
hypothesis seemingly strengthened by the volumetric hippocampal
data. However, the demonstration that food storers were,
indeed, better at spatial tasks was not readily come by. Most of the
early experiments, at least those comparing tit/chickadee species,
did not show compelling differences between the two groups, although
the trends were always in the predicted direction (e.g., Hilton &
Krebs, 1990; Krebs, Healy, & Shettleworth, 1990; Shettleworth,
Krebs, Healy, & Thomas, 1990).
However, work on the corvids was more promising (see Kamil et al.
this volume), and more recently, so have the results of experiments
on the Paridae. Food-storing tits and chickadees remember
spatial locations better than do nonstorers while the groups do not
differ in their ability to remember colours (e.g., Hampton & Shettleworth, 1996a, 1996b).
Food-storing coal tits Parus ater can also remember even
single locations for longer than do nonstoring great tits (see
Figure 7 - Biegler, McGregor, Krebs, & Healy, 2001; McGregor & Healy, 1999).
These results are consistent with predictions from the early
adaptationist hypothesis. It is unclear why the earlier
experiments did not show such clear differences. It is
possible that the explanation lies in the difficulty of the tasks
the birds had to solve; perhaps the earlier tasks were too simple
and the differences only became apparent when the task really taxed
their memory capabilities. If, as is supposed, the variation
in hippocampal underlies the difference in spatial cognition, it
should also be remembered that the nonstorers have a hippocampus,
which is far from vestigial. Additionally, they have to
remember some spatial information such as territory boundaries, nest
and rewarding food locations, so it was never going to be the case
that the comparison would be all or nothing. Yet again, it
seems that the choice of task is important. While some have
suggested that this implies that the adaptationist approach is at
fault, an alternative interpretation is that natural selection has
affected more subtle aspects of spatial cognition than was expected.
The issue of the specificity of experimental paradigm is a
substantial issue in the literature investigating differences in
spatial cognition in humans. Almost every lab uses a different
test to compare men and women, and although most have reached a
similar conclusion as to where the difference lies (i.e., in spatial
superiority by men over women, e.g., Moffat, Hampson, &
Hatzipantelis, 1998; Silverman et al., 2000), there is much less consensus in the views as
to the details of those differences, resulting in the plethora of
supposedly evolutionary hypotheses proposed as functional
explanations. In this field, unlike the comparisons in birds,
the adaptationist explanations have almost always followed
the demonstration of a sex difference. Much more comparative
data need to be collected, based on a priori predictions, in
order to differentiate which is the most likely among the possible
explanations currently proposed (for suggestions, see Jones,
Braithwaite, & Healy, 2003). There are also a number of studies that have found no
sex difference or a difference in which females seem to outperform
men (Duff & Hampson, 2001; James & Kimura, 1997; McBurney,
Gaulin, Devineni, & Adams, 1997; Postma, Izendoorn, & De Haan, 1998), which seems, yet again, to imply that
exposing cognitive differences is dependent on the specifics of the
task. This makes intuitive sense if one recognises that
spatial cognition is not a single entity but is made up of multiple
components, and in only some of these are men superior to women.
The issue of task dependence has not yet arisen in comparisons
between the sexes in rodents: males invariably outperform females
and certainly never perform more poorly, but these comparisons have
been made either in multi-arm dry mazes or in the Morris water maze.
Thus, there is vastly less variation in experimental design than in
the human studies.
|
|
 |
|
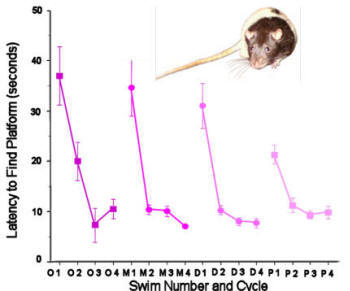
Figure 8. Female rats tested in a spatial working memory task in
a Morris water maze differed in their ability to learn the
location of the hidden platform across their oestrous cycle.
On oestrous days they required an extra swim before reaching
asymptotic performance. After Healy et al. (1999). |
A complication with all of the mammalian work,
to date, is that there is a clear role being played in spatial
cognition by hormones, especially sex and stress hormones.
These hormonal effects act both at an organisational level (during
prenatal development) and at an activational level (during the
animal's lifetime), with the former bringing about the most marked
effects (Williams, Barnett, & Meck, 1990; Williams & Meck, 1991).
Variation in the level of testosterone during an animal's time in
utero will have marked effects on that animal's spatial ability as
an adult, with the sexes being affected differently: if females
receive more testosterone than is usual, they will have better
spatial cognition, whereas extra testosterone for a male will lead to
decreased ability. Changes in oestrogens occurring across
menstrual or oestrous cycles cause short term fluctuations in
spatial ability (and in hippocampal morphology; see Figure 8), while
seasonal changes in testosterone cause changes in male spatial
ability: typically males have better spatial ability in the breeding
than in the non-breeding season (e.g., Galea, Kavaliers, Ossenkopp,
Innes, & Hargreaves, 1994a; Galea & McEwen, 1999;
Healy, Braham, & Braithwaite, 1999; Ormerod & Galea, 2003; Warren & Juraska, 1997).
Stress also affects spatial cognition in mammals, particularly
females who perform more poorly with increasing stress (Galea,
Saksida, Kavaliers, & Ossenkopp, 1994b; Kavaliers & Galea, 1995;
Kavaliers et al., 1996). It is not inconceivable that at least
some of the evidence for sex differences in spatial cognition in
mammals, then, is due to effects of stress caused by poor handling,
novel environments and so on, and not to natural selection.
Studies in which rats were handled extensively prior to testing are
among those that have not found a sex difference (e.g., Healy et
al., 1999; Perrot-Sinal, Kostenuik, Ossenkopp, & Kavaliers, 1996).
Neither species comparisons nor tests of
spatial learning and memory will enable complete avoidance of the
issue of possible sensory variation but they do reduce it
considerably. Ultimately, the only way to demonstrate that
variation in spatial ability is not explained by the capabilities of
exterior sensory structures is to conduct psychophysical or signal
detection experiments. Few, if any, of these have been carried
out on any of the species in which current comparative spatial
cognition is being examined. Such variation does exist,
even within species, often, but not always between the sexes (e.g.,
New World monkeys, Osorio, Smith, Vorobyev, & Buchanan-Smith, 2004;
e.g., blennies, White, Goncalves, Partridge, & Oliveira, 2004). There has even been a suggestion that such variation
can occur seasonally, within the same individual. It would be
useful, then, it seems, to do some of this type of investigation, if
only to show that a sensory explanation is insufficient.
One of the reasons that this sensory explanation has been accorded
less than, perhaps, its due weight, is that differences in cue
preference and in spatial cognition have been correlated with
variation in the hippocampus, the area of the brain commonly thought
to be predominant in the processing of spatial information.
Black-capped chickadees, coal and marsh tits, and jays all have
relatively larger hippocampal volumes than do great and blue tits,
dark-eyed juncos and jackdaws (Healy, Clayton, & Krebs, 1994; Krebs,
Sherry, Healy, Perry, & Vaccarino, 1989; Sherry, Vaccarino,
Buckenham, & Herz, 1989). Damage to the hippocampus in
food-storing birds, at least, does not impair their ability to solve
food finding tasks that require colour-vision/colour-learning but
birds with hippocampal lesions are unable to retrieve food stores
with more than chance accuracy, nor are they able to relocate
unmarked rewarded sites (Sherry & Vaccarino, 1989).
Hippocampal lesions also impair spatial cognition in both
black-capped chickadees and dark-eyed juncos, such that there are
not species differences following the lesion (Hampton & Shettleworth,
1996b), whereas such lesions do not impair memory for colour in
either species (Hampton & Shettleworth, 1996a). It is not
clear whether hippocampal lesions affect cue preferences.
I have reviewed some of the recent literature that
has incorporated adaptationist hypothesis testing as a way of
investigating variation in spatial cognition. Although there
are still relatively few studies to determine the extent to which
natural selection has shaped spatial cognition, there are sufficient
data to believe that the adaptationist framework continues to be
useful for formulating hypotheses (and therefore for producing
testable a priori
predictions) as to causes for variation in cognition. In this
way, it adds substantially to our understanding of comparative
spatial cognition.
Biegler, R., McGregor, A., Krebs, J.R., & Healy, S.D. (2001). A
larger hippocampus is associated with longer-lasting spatial memory.
Proceedings of the National Academy of Sciences of the United
States of America, 98, 6941-6944.
Biro,
D., Meade, J., & Guilford, T. (2004). Familiar route loyalty implies
visual pilotage in the homing pigeon. Proceedings of the National
Academy of Sciences of the United States of America, 101,
17440-17443.
Braithwaite, V.A., & Girvan, J.R. (2003). Use of water flow
direction to provide spatial information in a small-scale
orientation task. Journal of Fish Biology, 63, 74-83.
Brodbeck, D.R. (1994). Memory for spatial and local cues - A
comparison of a storing and a non-storing species. Animal
Learning & Behavior, 22, 119-133.
Brodbeck, D.R. & Shettleworth, S.J. (1995). Matching location and
color of a compound stimulus - comparison of a food-storing and a
nonstoring bird species. Journal of Experimental Psychology:
Animal Behavior Processes, 21, 64-77.
Clayton, N.S., & Krebs, J.R. (1994). Memory for
spatial and object-specific cues in food-storing and non-storing
birds. Journal of Comparative Physiology A: Sensory Neural and
Behavioral Physiology, 174, 371-379.
Cristol, D.A., Reynolds, E.B., Leclerc, J.E.,
Donner, A.H., Farabaugh, C.S., & Ziegenfus, C.W.S. (2003). Migratory
dark-eyed juncos, Junco hyemalis, have better spatial memory
and denser hippocampal neurons than nonmigratory conspecifics.
Animal Behaviour, 66, 317-328.
Crystal, J.D. & Shettleworth, S.J. (1994).
Spatial list learning in black-capped chickadees. Animal Learning
& Behavior, 22, 77-83.
Dabbs, J.M., Chang, E.L., Strong, R.A., & Milun,
R. (1998). Spatial ability, navigation strategy, and geographic
knowledge among men and women. Evolution and Human Behavior,
19, 89-98.
Dawkins, M.S., Guilford, T., Braithwaite, V.A., &
Krebs, J.R. (1996). Discrimination and recognition of photographs of
places by homing pigeons. Behavioural Processes, 36, 27-38.
de Perera, T.B. (2004). Fish can encode order in
their spatial map. Proceedings of the Royal Society of London,
Series B, 271, 2131-2134.
Duff, S.J., & Hampson, E. (2001). A sex
difference on a novel spatial working memory task in humans.
Brain and Cognition, 47, 470-493.
Galea, L.A.M., Kavaliers, M., Ossenkopp, K.P.,
Innes, D., & Hargreaves, E.L. (1994a). Sexually dimorphic spatial
learning varies seasonally in two populations of deer mice. Brain
Research, 635, 18-26.
Galea, L.A.M., & McEwen, B.S. (1999). Sex and
seasonal differences in the rate of cell proliferation in the
dentate gyrus of adult wild meadow voles. Neuroscience, 89,
955-964.
Galea, L.A.M., Saksida, L., Kavaliers, M., &
Ossenkopp, K.P. (1994b). Naloxone facilitates spatial learning in a
water-maze task in female, but not male, adult non-breeding meadow
voles. Pharmacology Biochemistry and Behavior, 47,
265-271.
Garrison, J.S.E., & Gass, C.L. (1999). Response
of a traplining hummingbird to changes in nectar availability.
Behavioral Ecology, 10, 714-725.
Gass, C.L., & Garrison, J.S.E. (1999). Energy
regulation by traplining hummingbirds. Functional Ecology,
13, 438-492.
Gibson, B.M., & Shettleworth, S.J. (2003).
Competition among spatial cues in a naturalistic food-carrying task.
Learning & Behavior, 31, 143-159.
Girvan, J.R. & Braithwaite, V.A. (1998).
Population differences in spatial learning in three-spined
sticklebacks. Proceedings of the Royal Society of London, Series
B, 265, 913-918.
Girvan, J.R., & Braithwaite, V.A. (2000).
Orientation behaviour in sticklebacks: Modified by experience or
population specific? Behaviour, 137, 833-843.
Grant, K.A. (1966). A hypothesis concerning the
prevalence of red coloration in California hummingbird flowers.
American Naturalist, 100, 85-97.
Guilford, T., Roberts, S., Biro, D., & Rezek, L.
(2004). Positional entropy during pigeon homing II: Navigational
interpretation of Bayesian latent state models. Journal of
Theoretical Biology, 227, 25-38.
Hampton, R.R., Healy, S.D., Shettleworth, S.J., &
Kamil, A.C. (2002). 'Neuroecologists' are not made of straw.
Trends in Cognitive Sciences, 6, 6-7.
Hampton, R.R., & Shettleworth, S.J. (1996a).
Hippocampal lesions impair memory for location but not color in
passerine birds. Behavioral Neuroscience, 110,
831-835.
Hampton, R.R., & Shettleworth, S.J. (1996b).
Hippocampus and memory in a food-storing and in a nonstoring bird
species. Behavioral Neuroscience, 110, 946-964.
Healy, S.D., Braham, S.R., & Braithwaite, V.A.
(1999). Spatial working memory in rats: No differences between the
sexes. Proceedings of the Royal Society of London, Series B,
266, 2303-2308.
Healy, S.D., Clayton, N.S., & Krebs, J.R. (1994).
Development of hippocampal specialization in 2 species of tit (Parus
spp). Behavioural Brain Research, 61, 23-28.
Healy, S.D. & Hurly, T.A. (1995). Spatial memory in rufous
hummingbirds (Selasphorus rufus): A field test. Animal
Learning & Behavior, 23, 63-68.
Healy, S.D., & Hurly, T.A. (1998). Rufous
hummingbirds' (Selasphorus rufus) memory for flowers:
Patterns or actual spatial locations? Journal of Experimental
Psychology: Animal Behavior Processes, 24, 396-404.
Hilton, S.C., & Krebs, J. R. (1990). Spatial
memory of four species of Parus: Performance in an open-field
analogue of a radial maze. The Quarterly Journal of Experimental
Psychology, 42B, 345-368.
Hodgson, Z. & Healy, S. D. (2005). Preference for
spatial cues in a non-storing songbird species. Animal Cognition,
8, 211-213.
Hurly, T.A. & Healy, S.D. (1996). Memory for
flowers in rufous hummingbirds: Location or local visual cues?
Animal Behaviour, 51, 1149-1157.
Hurly, T.A. & Healy, S.D. (2002). Cue learning by
rufous hummingbirds (Selasphorus rufus). Journal of
Experimental Psychology: Animal Behavior Processes, 28,
209-223.
James, T.W., & Kimura, D. (1997). Sex differences
in remembering the locations of objects in an array: Location-shifts
versus location-exchanges. Evolution and Human Behavior,
18, 155-163.
Jones, C.M., Braithwaite,
V.A., & Healy, S.D. (2003). The evolution of sex differences in
spatial ability. Behavioral Neuroscience, 117, 403-411.
Kanit, L., Taskiran, D., Furedy, J. J., Kulali,
B., McDonald, R. & Pogun, S. (1998). Nicotine interacts with sex in
affecting rat choice between "look-out" and "navigational" cognitive
styles in the Morris water maze place learning task. Brain
Research Bulletin, 46, 441-445.
Kanit, L., Taskiran, D., Yilmaz, O. A., Balkan,
B., Demirgoren, S., Furedy, J.J., & Pogun, S. (2000). Sexually
dimorphic cognitive style in rats emerges after puberty. Brain
Research Bulletin, 52, 243-248.
Kavaliers, M., & Galea, L.A.M. (1995). Sex
differences in the expression and antagonism of swim stress-induced
analgesia in deer mice vary with the breeding season. Pain,
63, 327-334.
Kavaliers, M., Ossenkopp, K.P., Prato, F.S.,
Innes, D.G.L., Galea, L.A.M., Kinsella, D.M., & Perrot-Sinal, T.S.
(1996). Spatial learning in deer mice: Sex differences and the
effects of endogenous opioids and 60 Hz magnetic fields. Journal
of Comparative Physiology A, 179, 715-724.
Keiser, J.T., Ziegenfus, C.W.S. & Cristol, D.A.
(2005). Homing success of migrant versus nonmigrant dark-eyed juncos
(Junco hyemalis). Auk, 122, 608-617.
Krebs, J.R., Healy, S.D., & Shettleworth, S.J.
(1990). Spatial memory of Paridae: Comparison of a storing and a
non-storing species, the coal tit Parus ater, and the great
tit, P.major. Animal Behaviour, 39, 1127-1137.
Krebs, J.R., Sherry, D.F., Healy, S.D., Perry,
V.H., & Vaccarino, A.L. (1989). Hippocampal specialization of
food-storing birds. Proceedings of the National Academy of
Sciences of the United States of America, 86, 1388-1392.
MacFadden, A., Elias, L., & Saucier, D. (2003).Males and females
scan maps similarly, but give directions differently. Brain and
Cognition, 53, 297-300.
Macphail, E.M., & Bolhuis, J.J. (2001). The evolution of
intelligence: Adaptive specializations versus general
process. Biological Reviews, 76, 341-364.
McBurney, D.H., Gaulin, S.J.C., Devineni, T. & Adams, C. (1997).
Superior spatial memory of women: Stronger evidence for the
gathering hypothesis. Evolution and Human Behavior, 18,
165-174.
McGregor, A., Good, M.A. & Pearce, J.M. (2004). Absence of an
interaction between navigational strategies based on local and
distal landmarks. Journal of Experimental Psychology: Animal
Behavior Processes, 30, 34-44.
McGregor, A. & Healy, S.D. (1999). Spatial accuracy in food-storing
and nonstoring birds. Animal Behaviour, 58, 727-734.
Miller, R.S., Tamm, S., Sutherland, G.D., & Gass, C.L. (1985). Cues
for orientation in hummingbird foraging: Color and position.
Canadian Journal of Zoology, 63, 18-21.
Moffat, S.D., Hampson, E. & Hatzipantelis, M. (1998). Navigation in
a "virtual" maze: Sex differences and correlation with psychometric
measures of spatial ability in humans. Evolution and Human
Behavior, 19, 73-87.
Odling-Smee, L., & Braithwaite, V. A. (2003). The influence of
habitat stability on landmark use during spatial learning in the
three-spined stickleback. Animal Behaviour, 65,
701-707.
Ormerod, B.K., & Galea, L.A.M. (2003). Reproductive status
influences the survival of new cells in the dentate gyrus of adult
male meadow voles. Neuroscience Letters, 346, 25-28.
Osorio, D., Smith, A.C., Vorobyev, M., & Buchanan-Smith, H.M.
(2004). Detection of fruit and the selection of primate visual
pigments for color vision. American Naturalist, 164,
696-708.
Perrot-Sinal, T.S., Kostenuik, M.A., Ossenkopp, K.-P., & Kavaliers,
M. (1996). Sex differences in performance in the Morris water maze
and the effects of initial nonstationary hidden platform training.
Behavioral Neuroscience, 110, 1309-1320.
Postma, A., Izendoorn, R., & De Haan, E.H.F. (1998). Sex differences
in object location memory. Brain and Cognition, 36,
334-345.
Rehkamper, G., Haase, E., & Frahm, H.D. (1988). Allometric
comparison of brain weight and brain structure volumes in different
breeds of the domestic pigeon. Brain, Behaviour and Evolution,
31, 141-149.
Sandstrom, N.J., Kaufman, J., & Huettel, S.A. (1998). Males and
females use different distal cues in a virtual environment
navigation task. Cognitive Brain Research, 6, 351-360.
Schmidt-Koenig, K., & Walcott, C. (1978). Tracks of pigeons homing
with frosted lenses. Animal Behaviour, 26, 480-486.
Sherry, D. F., & Vaccarino, A. L. (1989). Hippocampus and memory for
food caches in black-capped chickadees. Behavioral Neuroscience,
103, 308-318.
Sherry, D.F., Vaccarino, A.L., Buckenham, K., & Herz, R.S. (1989).
The hippocampal complex of food-storing birds. Brain, Behavior,
and Evolution, 34, 308-317.
Shettleworth, S.J., Krebs, J.R., Healy, S.D., & Thomas, C.M. (1990).
Spatial memory of food-storing tits (Parus ater and P.
atricapillus): Comparison of storing and nonstoring tasks.
Journal of Comparative Psychology, 104, 71-81.
Silverman, I., Choi, J., Mackewn, A., Fisher, M., Moro, J., &
Olshansky, E. (2000). Evolved mechanisms underlying wayfinding:
Further studies on the hunter-gatherer theory of spatial sex
differences. Evolution and Human Behavior, 21,
201-213.
Spencer, C., & Weetman, M. (1981). The microgenesis of cognitive
maps - A longitudinal-study of new residents of an urban area.
Transactions of the Institute of British Geographers, 6,
375-384.
Warren, S. G., & Juraska, J.M. (1997). Spatial and nonspatial
learning across the rat estrous cycle. Behavioral Neuroscience,
111, 259-266.
White, E.M., Goncalves, D.M., Partridge, J.C., & Oliveira, R.F.
(2004). Vision and visual variation in the peacock blenny.
Journal of Fish Biology, 65, 227-250.
Wilkie, D.M., Willson, R.J., & Kardal, S. (1989). Pigeons
discriminate pictures of a geographic location. Animal Learning &
Behavior, 17, 163-171.
Williams, C.L., Barnett, A. M., & Meck, W.H. (1990). Organizational
effects of gonadal secretions on sexual differentiation in spatial
memory. Behavioral Neuroscience, 104, 84-97.
Williams, C.L., & Meck, W.H. (1991). The organizational effects of
gonadal steroids on sexually dimorphic spatial ability.
Psychoneuroendocrinology, 16, 155-176.
©2006 All copyrights for the individual chapters are retained by the
authors. All other material in this book is copyrighted by the
editor, unless noted otherwise. If there has been an error with
regards to unacknowledged copyrighted material, please contact the
editor immediately so that this can be corrected. Permissions for
using material in this book should be sent to the editor.