|
|
 |
|
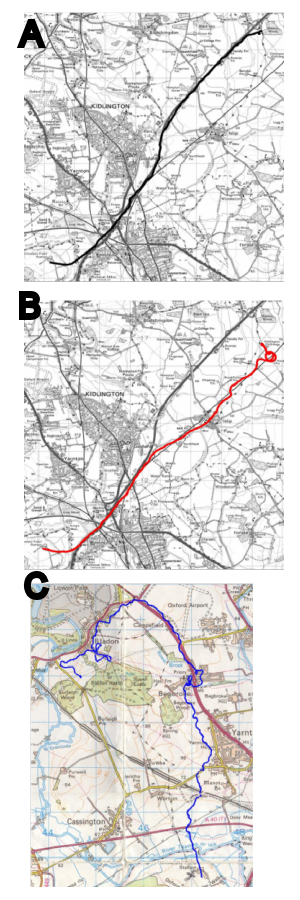
Figure 5. Use of spatial rather than visual cues by
hummingbirds in an array of flowers, three of which were
rewarded (marked by heavy black lines). Birds were trained
with arrays as seen in the left hand column. The arrays
were then moved 2m and birds trained with rewarded flowers in
the same or new positions, bearing the same or new colour
patterns. Birds did not appear to have learned, or to use,
the colour patterns of the flowers in Phase 1 when learning
which were the rewarded flowers in Phase 2. After Hurly &
Healy (2002).
|
|
|
 |
|
|
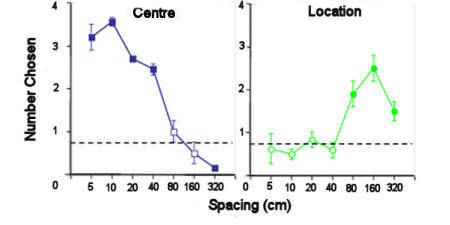
Figure 6. Rufous hummingbirds were trained to
feed from the central flower in an array of flowers and the
array was then moved such that the new centre was in the same
location as one of the outer flowers. When the flowers in
the array were 40cm apart or closer, the birds returned to the
flower in the centre. When they were further apart, the
birds returned to flowers in the original location. After
Healy & Hurly (1998).
|
A second way to make comparisons of spatial cognition
is to assess learning and memory, rather than cue preferences.
Ideally, one would have some understanding of the cue preferences
when comparing learning and memory, although examining factors such
as the ease with which animals reach a criterion before performance
is assessed may provide this to some extent.
Tests of cognitive ability followed the
cue preference tests in rufous hummingbirds. The question was then, what would one
predict these birds to use for ready remembrance of flowers if one
was to follow an adaptationist train of thought? Although the
hummingbirds prefer spatial (an arrangement of unspecified visual
cues) over beacon-like, visual cues does not mean the birds should
not learn and remember the visual information provided by flowers.
However, if the bird has to remember a flower for several hours, it
seems more likely that the flower's visual features are far more
likely to have altered than its spatial location. In fact, it
appears that even over the course of far shorter periods of time,
birds trained to find several neighbouring rewarding flowers in an
array failed to show any evidence of having learned the visual cues
of the flowers when trained on a similar array that had been moved
2m from the original location (see Figure 5, Hurly & Healy, 2002).
It made no difference to the birds' learning which were the rewarded
flowers in the new array, whether the flowers in this second array
were of the same or different colour patterns as those in the first
array, as long as the flowers were in the same relative positions.
Not until the shape of the test array, as well as its location, were
altered from the training situation did it become clear that the
birds had learned the colour patterns of the flowers in addition to
their positions in the array.
If the array is moved such that part of it overlays the
location of the original, the birds can be shown to encode which are
the rewarded flowers relative to the distance among the flowers:
flowers that are further than 40cm apart are encoded relative to
cues surrounding the array, while flowers that are closer together
than this are encoded relative to each other (see Figure 6, Healy &
Hurly, 1998). With regard to the adaptationist model, it is not
clear that these latter results would have been predicted, even with
greater knowledge of the animal's behaviour. However, it is
the case that without the original ecological rationale that
hummingbird foraging might be a fertile field for investigation into
spatial learning and memory, it is unlikely that findings such as
the scale of cue use would have come to light. Variation in
hummingbird foraging style (territory defence and traplining, where
animals travel a repeated route around widely spaced food sites) might
provide further insights into both the ways that animals use visual
and spatial information as well as offer the possibility that the
different foraging styles are accompanied by differences in
cognition. Traplining hummingbirds forage in a markedly
different way from that of territorial hummingbirds. For example,
they feed on flowers that decrease nectar production during the day,
a pattern that the birds mimic in their visitation rates whereas
territorial birds feed at a relatively constant rate throughout the
day (Garrison & Gass, 1999; Gass & Garrison, 1999). As a
result, traplining birds may be particularly good, for example, at
learning the sequence of places (Crystal & Shettleworth, 1994), or
may only encode food sites in a sequence (de Perera, 2004), rather
than in some more flexible way that would allow birds to access
sites at any time from any place (a.k.a. a cognitive map).
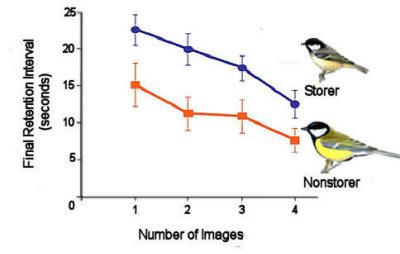 |
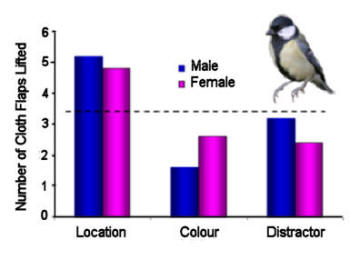
Figure 7. Coal tits (a food storing species) and
great tits (a nonstoring species) were trained and tested on a
spatial delayed non-matching-to-sample task presented on a touch
screen in which the number of samples, the duration of the
retention interval and the distance the choice images were apart
were all manipulated. The outcome was that coal tits were
better at remembering even a single location for longer than
were the great tits. After Biegler et al. (2001).
|
Another example in which cue preference was known, food storing,
provided an ecological rationale for the hypothesis that
scatter-storing (as opposed to larder storing) animals, with their
greater dependence on spatial memory for successful retrieval of
their stores, would outperform nonstorers on spatial memory tasks, a
hypothesis seemingly strengthened by the volumetric hippocampal
data. However, the demonstration that food storers were,
indeed, better at spatial tasks was not readily come by. Most of the
early experiments, at least those comparing tit/chickadee species,
did not show compelling differences between the two groups, although
the trends were always in the predicted direction (e.g., Hilton &
Krebs, 1990; Krebs, Healy, & Shettleworth, 1990; Shettleworth,
Krebs, Healy, & Thomas, 1990).
However, work on the corvids was more promising (see Kamil et al.
this volume), and more recently, so have the results of experiments
on the Paridae. Food-storing tits and chickadees remember
spatial locations better than do nonstorers while the groups do not
differ in their ability to remember colours (e.g., Hampton & Shettleworth, 1996a, 1996b).
Food-storing coal tits Parus ater can also remember even
single locations for longer than do nonstoring great tits (see
Figure 7 - Biegler, McGregor, Krebs, & Healy, 2001; McGregor & Healy, 1999).
These results are consistent with predictions from the early
adaptationist hypothesis. It is unclear why the earlier
experiments did not show such clear differences. It is
possible that the explanation lies in the difficulty of the tasks
the birds had to solve; perhaps the earlier tasks were too simple
and the differences only became apparent when the task really taxed
their memory capabilities. If, as is supposed, the variation
in hippocampal underlies the difference in spatial cognition, it
should also be remembered that the nonstorers have a hippocampus,
which is far from vestigial. Additionally, they have to
remember some spatial information such as territory boundaries, nest
and rewarding food locations, so it was never going to be the case
that the comparison would be all or nothing. Yet again, it
seems that the choice of task is important. While some have
suggested that this implies that the adaptationist approach is at
fault, an alternative interpretation is that natural selection has
affected more subtle aspects of spatial cognition than was expected.
The issue of the specificity of experimental paradigm is a
substantial issue in the literature investigating differences in
spatial cognition in humans. Almost every lab uses a different
test to compare men and women, and although most have reached a
similar conclusion as to where the difference lies (i.e., in spatial
superiority by men over women, e.g., Moffat, Hampson, &
Hatzipantelis, 1998; Silverman et al., 2000), there is much less consensus in the views as
to the details of those differences, resulting in the plethora of
supposedly evolutionary hypotheses proposed as functional
explanations. In this field, unlike the comparisons in birds,
the adaptationist explanations have almost always followed
the demonstration of a sex difference. Much more comparative
data need to be collected, based on a priori predictions, in
order to differentiate which is the most likely among the possible
explanations currently proposed (for suggestions, see Jones,
Braithwaite, & Healy, 2003). There are also a number of studies that have found no
sex difference or a difference in which females seem to outperform
men (Duff & Hampson, 2001; James & Kimura, 1997; McBurney,
Gaulin, Devineni, & Adams, 1997; Postma, Izendoorn, & De Haan, 1998), which seems, yet again, to imply that
exposing cognitive differences is dependent on the specifics of the
task. This makes intuitive sense if one recognises that
spatial cognition is not a single entity but is made up of multiple
components, and in only some of these are men superior to women.
The issue of task dependence has not yet arisen in comparisons
between the sexes in rodents: males invariably outperform females
and certainly never perform more poorly, but these comparisons have
been made either in multi-arm dry mazes or in the Morris water maze.
Thus, there is vastly less variation in experimental design than in
the human studies.
A complication with all of the mammalian work,
to date, is that there is a clear role being played in spatial
cognition by hormones, especially sex and stress hormones.
These hormonal effects act both at an organisational level (during
prenatal development) and at an activational level (during the
animal's lifetime), with the former bringing about the most marked
effects (Williams, Barnett, & Meck, 1990; Williams & Meck, 1991).
Variation in the level of testosterone during an animal's time in
utero will have marked effects on that animal's spatial ability as
an adult, with the sexes being affected differently: if females
receive more testosterone than is usual, they will have better
spatial cognition, whereas extra testosterone for a male will lead to
decreased ability. Changes in oestrogens occurring across
menstrual or oestrous cycles cause short term fluctuations in
spatial ability (and in hippocampal morphology; see Figure 8), while
seasonal changes in testosterone cause changes in male spatial
ability: typically males have better spatial ability in the breeding
than in the non-breeding season (e.g., Galea, Kavaliers, Ossenkopp,
Innes, & Hargreaves, 1994a; Galea & McEwen, 1999;
Healy, Braham, & Braithwaite, 1999; Ormerod & Galea, 2003; Warren & Juraska, 1997).
|
|
 |
|
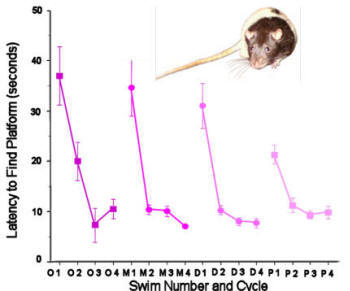
Figure 8. Female rats tested in a spatial working memory task in
a Morris water maze differed in their ability to learn the
location of the hidden platform across their oestrous cycle.
On oestrous days they required an extra swim before reaching
asymptotic performance. After Healy et al. (1999). |
Stress also affects spatial cognition in mammals, particularly
females who perform more poorly with increasing stress (Galea,
Saksida, Kavaliers, & Ossenkopp, 1994b; Kavaliers & Galea, 1995;
Kavaliers et al., 1996). It is not inconceivable that at least
some of the evidence for sex differences in spatial cognition in
mammals, then, is due to effects of stress caused by poor handling,
novel environments and so on, and not to natural selection.
Studies in which rats were handled extensively prior to testing are
among those that have not found a sex difference (e.g., Healy et
al., 1999; Perrot-Sinal, Kostenuik, Ossenkopp, & Kavaliers, 1996).
Neither species comparisons nor tests of
spatial learning and memory will enable complete avoidance of the
issue of possible sensory variation but they do reduce it
considerably. Ultimately, the only way to demonstrate that
variation in spatial ability is not explained by the capabilities of
exterior sensory structures is to conduct psychophysical or signal
detection experiments. Few, if any, of these have been carried
out on any of the species in which current comparative spatial
cognition is being examined. Such variation does exist,
even within species, often, but not always between the sexes (e.g.,
New World monkeys, Osorio, Smith, Vorobyev, & Buchanan-Smith, 2004;
e.g., blennies, White, Goncalves, Partridge, & Oliveira, 2004). There has even been a suggestion that such variation
can occur seasonally, within the same individual. It would be
useful, then, it seems, to do some of this type of investigation, if
only to show that a sensory explanation is insufficient.
One of the reasons that this sensory explanation has been accorded
less than, perhaps, its due weight, is that differences in cue
preference and in spatial cognition have been correlated with
variation in the hippocampus, the area of the brain commonly thought
to be predominant in the processing of spatial information.
Black-capped chickadees, coal and marsh tits, and jays all have
relatively larger hippocampal volumes than do great and blue tits,
dark-eyed juncos and jackdaws (Healy, Clayton, & Krebs, 1994; Krebs,
Sherry, Healy, Perry, & Vaccarino, 1989; Sherry, Vaccarino,
Buckenham, & Herz, 1989). Damage to the hippocampus in
food-storing birds, at least, does not impair their ability to solve
food finding tasks that require colour-vision/colour-learning but
birds with hippocampal lesions are unable to retrieve food stores
with more than chance accuracy, nor are they able to relocate
unmarked rewarded sites (Sherry & Vaccarino, 1989).
Hippocampal lesions also impair spatial cognition in both
black-capped chickadees and dark-eyed juncos, such that there are
not species differences following the lesion (Hampton & Shettleworth,
1996b), whereas such lesions do not impair memory for colour in
either species (Hampton & Shettleworth, 1996a). It is not
clear whether hippocampal lesions affect cue preferences.
I have reviewed some of the recent literature that
has incorporated adaptationist hypothesis testing as a way of
investigating variation in spatial cognition. Although there
are still relatively few studies to determine the extent to which
natural selection has shaped spatial cognition, there are sufficient
data to believe that the adaptationist framework continues to be
useful for formulating hypotheses (and therefore for producing
testable a priori
predictions) as to causes for variation in cognition. In this
way, it adds substantially to our understanding of comparative
spatial cognition.
Biegler, R., McGregor, A., Krebs, J.R., & Healy, S.D. (2001). A
larger hippocampus is associated with longer-lasting spatial memory.
Proceedings of the National Academy of Sciences of the United
States of America, 98, 6941-6944.
Biro,
D., Meade, J., & Guilford, T. (2004). Familiar route loyalty implies
visual pilotage in the homing pigeon. Proceedings of the National
Academy of Sciences of the United States of America, 101,
17440-17443.
Braithwaite, V.A., & Girvan, J.R. (2003). Use of water flow
direction to provide spatial information in a small-scale
orientation task. Journal of Fish Biology, 63, 74-83.
Brodbeck, D.R. (1994). Memory for spatial and local cues - A
comparison of a storing and a non-storing species. Animal
Learning & Behavior, 22, 119-133.
Brodbeck, D.R. & Shettleworth, S.J. (1995). Matching location and
color of a compound stimulus - comparison of a food-storing and a
nonstoring bird species. Journal of Experimental Psychology:
Animal Behavior Processes, 21, 64-77.
Clayton, N.S., & Krebs, J.R. (1994). Memory for
spatial and object-specific cues in food-storing and non-storing
birds. Journal of Comparative Physiology A: Sensory Neural and
Behavioral Physiology, 174, 371-379.
Cristol, D.A., Reynolds, E.B., Leclerc, J.E.,
Donner, A.H., Farabaugh, C.S., & Ziegenfus, C.W.S. (2003). Migratory
dark-eyed juncos, Junco hyemalis, have better spatial memory
and denser hippocampal neurons than nonmigratory conspecifics.
Animal Behaviour, 66, 317-328.
Crystal, J.D. & Shettleworth, S.J. (1994).
Spatial list learning in black-capped chickadees. Animal Learning
& Behavior, 22, 77-83.
Dabbs, J.M., Chang, E.L., Strong, R.A., & Milun,
R. (1998). Spatial ability, navigation strategy, and geographic
knowledge among men and women. Evolution and Human Behavior,
19, 89-98.
Dawkins, M.S., Guilford, T., Braithwaite, V.A., &
Krebs, J.R. (1996). Discrimination and recognition of photographs of
places by homing pigeons. Behavioural Processes, 36, 27-38.
de Perera, T.B. (2004). Fish can encode order in
their spatial map. Proceedings of the Royal Society of London,
Series B, 271, 2131-2134.
Duff, S.J., & Hampson, E. (2001). A sex
difference on a novel spatial working memory task in humans.
Brain and Cognition, 47, 470-493.
Galea, L.A.M., Kavaliers, M., Ossenkopp, K.P.,
Innes, D., & Hargreaves, E.L. (1994a). Sexually dimorphic spatial
learning varies seasonally in two populations of deer mice. Brain
Research, 635, 18-26.
Galea, L.A.M., & McEwen, B.S. (1999). Sex and
seasonal differences in the rate of cell proliferation in the
dentate gyrus of adult wild meadow voles. Neuroscience, 89,
955-964.
Galea, L.A.M., Saksida, L., Kavaliers, M., &
Ossenkopp, K.P. (1994b). Naloxone facilitates spatial learning in a
water-maze task in female, but not male, adult non-breeding meadow
voles. Pharmacology Biochemistry and Behavior, 47,
265-271.
Garrison, J.S.E., & Gass, C.L. (1999). Response
of a traplining hummingbird to changes in nectar availability.
Behavioral Ecology, 10, 714-725.
Gass, C.L., & Garrison, J.S.E. (1999). Energy
regulation by traplining hummingbirds. Functional Ecology,
13, 438-492.
Gibson, B.M., & Shettleworth, S.J. (2003).
Competition among spatial cues in a naturalistic food-carrying task.
Learning & Behavior, 31, 143-159.
Girvan, J.R. & Braithwaite, V.A. (1998).
Population differences in spatial learning in three-spined
sticklebacks. Proceedings of the Royal Society of London, Series
B, 265, 913-918.
Girvan, J.R., & Braithwaite, V.A. (2000).
Orientation behaviour in sticklebacks: Modified by experience or
population specific? Behaviour, 137, 833-843.
Grant, K.A. (1966). A hypothesis concerning the
prevalence of red coloration in California hummingbird flowers.
American Naturalist, 100, 85-97.
Guilford, T., Roberts, S., Biro, D., & Rezek, L.
(2004). Positional entropy during pigeon homing II: Navigational
interpretation of Bayesian latent state models. Journal of
Theoretical Biology, 227, 25-38.
Hampton, R.R., Healy, S.D., Shettleworth, S.J., &
Kamil, A.C. (2002). 'Neuroecologists' are not made of straw.
Trends in Cognitive Sciences, 6, 6-7.
Hampton, R.R., & Shettleworth, S.J. (1996a).
Hippocampal lesions impair memory for location but not color in
passerine birds. Behavioral Neuroscience, 110,
831-835.
Hampton, R.R., & Shettleworth, S.J. (1996b).
Hippocampus and memory in a food-storing and in a nonstoring bird
species. Behavioral Neuroscience, 110, 946-964.
Healy, S.D., Braham, S.R., & Braithwaite, V.A.
(1999). Spatial working memory in rats: No differences between the
sexes. Proceedings of the Royal Society of London, Series B,
266, 2303-2308.
Healy, S.D., Clayton, N.S., & Krebs, J.R. (1994).
Development of hippocampal specialization in 2 species of tit (Parus
spp). Behavioural Brain Research, 61, 23-28.
Healy, S.D. & Hurly, T.A. (1995). Spatial memory in rufous
hummingbirds (Selasphorus rufus): A field test. Animal
Learning & Behavior, 23, 63-68.
Healy, S.D., & Hurly, T.A. (1998). Rufous
hummingbirds' (Selasphorus rufus) memory for flowers:
Patterns or actual spatial locations? Journal of Experimental
Psychology: Animal Behavior Processes, 24, 396-404.
Hilton, S.C., & Krebs, J. R. (1990). Spatial
memory of four species of Parus: Performance in an open-field
analogue of a radial maze. The Quarterly Journal of Experimental
Psychology, 42B, 345-368.
Hodgson, Z. & Healy, S. D. (2005). Preference for
spatial cues in a non-storing songbird species. Animal Cognition,
8, 211-213.
Hurly, T.A. & Healy, S.D. (1996). Memory for
flowers in rufous hummingbirds: Location or local visual cues?
Animal Behaviour, 51, 1149-1157.
Hurly, T.A. & Healy, S.D. (2002). Cue learning by
rufous hummingbirds (Selasphorus rufus). Journal of
Experimental Psychology: Animal Behavior Processes, 28,
209-223.
James, T.W., & Kimura, D. (1997). Sex differences
in remembering the locations of objects in an array: Location-shifts
versus location-exchanges. Evolution and Human Behavior,
18, 155-163.
Jones, C.M., Braithwaite,
V.A., & Healy, S.D. (2003). The evolution of sex differences in
spatial ability. Behavioral Neuroscience, 117, 403-411.
Kanit, L., Taskiran, D., Furedy, J. J., Kulali,
B., McDonald, R. & Pogun, S. (1998). Nicotine interacts with sex in
affecting rat choice between "look-out" and "navigational" cognitive
styles in the Morris water maze place learning task. Brain
Research Bulletin, 46, 441-445.
Kanit, L., Taskiran, D., Yilmaz, O. A., Balkan,
B., Demirgoren, S., Furedy, J.J., & Pogun, S. (2000). Sexually
dimorphic cognitive style in rats emerges after puberty. Brain
Research Bulletin, 52, 243-248.
Kavaliers, M., & Galea, L.A.M. (1995). Sex
differences in the expression and antagonism of swim stress-induced
analgesia in deer mice vary with the breeding season. Pain,
63, 327-334.
Kavaliers, M., Ossenkopp, K.P., Prato, F.S.,
Innes, D.G.L., Galea, L.A.M., Kinsella, D.M., & Perrot-Sinal, T.S.
(1996). Spatial learning in deer mice: Sex differences and the
effects of endogenous opioids and 60 Hz magnetic fields. Journal
of Comparative Physiology A, 179, 715-724.
Keiser, J.T., Ziegenfus, C.W.S. & Cristol, D.A.
(2005). Homing success of migrant versus nonmigrant dark-eyed juncos
(Junco hyemalis). Auk, 122, 608-617.
Krebs, J.R., Healy, S.D., & Shettleworth, S.J.
(1990). Spatial memory of Paridae: Comparison of a storing and a
non-storing species, the coal tit Parus ater, and the great
tit, P.major. Animal Behaviour, 39, 1127-1137.
Krebs, J.R., Sherry, D.F., Healy, S.D., Perry,
V.H., & Vaccarino, A.L. (1989). Hippocampal specialization of
food-storing birds. Proceedings of the National Academy of
Sciences of the United States of America, 86, 1388-1392.
MacFadden, A., Elias, L., & Saucier, D. (2003).Males and females
scan maps similarly, but give directions differently. Brain and
Cognition, 53, 297-300.
Macphail, E.M., & Bolhuis, J.J. (2001). The evolution of
intelligence: Adaptive specializations versus general
process. Biological Reviews, 76, 341-364.
McBurney, D.H., Gaulin, S.J.C., Devineni, T. & Adams, C. (1997).
Superior spatial memory of women: Stronger evidence for the
gathering hypothesis. Evolution and Human Behavior, 18,
165-174.
McGregor, A., Good, M.A. & Pearce, J.M. (2004). Absence of an
interaction between navigational strategies based on local and
distal landmarks. Journal of Experimental Psychology: Animal
Behavior Processes, 30, 34-44.
McGregor, A. & Healy, S.D. (1999). Spatial accuracy in food-storing
and nonstoring birds. Animal Behaviour, 58, 727-734.
Miller, R.S., Tamm, S., Sutherland, G.D., & Gass, C.L. (1985). Cues
for orientation in hummingbird foraging: Color and position.
Canadian Journal of Zoology, 63, 18-21.
Moffat, S.D., Hampson, E. & Hatzipantelis, M. (1998). Navigation in
a "virtual" maze: Sex differences and correlation with psychometric
measures of spatial ability in humans. Evolution and Human
Behavior, 19, 73-87.
Odling-Smee, L., & Braithwaite, V. A. (2003). The influence of
habitat stability on landmark use during spatial learning in the
three-spined stickleback. Animal Behaviour, 65,
701-707.
Ormerod, B.K., & Galea, L.A.M. (2003). Reproductive status
influences the survival of new cells in the dentate gyrus of adult
male meadow voles. Neuroscience Letters, 346, 25-28.
Osorio, D., Smith, A.C., Vorobyev, M., & Buchanan-Smith, H.M.
(2004). Detection of fruit and the selection of primate visual
pigments for color vision. American Naturalist, 164,
696-708.
Perrot-Sinal, T.S., Kostenuik, M.A., Ossenkopp, K.-P., & Kavaliers,
M. (1996). Sex differences in performance in the Morris water maze
and the effects of initial nonstationary hidden platform training.
Behavioral Neuroscience, 110, 1309-1320.
Postma, A., Izendoorn, R., & De Haan, E.H.F. (1998). Sex differences
in object location memory. Brain and Cognition, 36,
334-345.
Rehkamper, G., Haase, E., & Frahm, H.D. (1988). Allometric
comparison of brain weight and brain structure volumes in different
breeds of the domestic pigeon. Brain, Behaviour and Evolution,
31, 141-149.
Sandstrom, N.J., Kaufman, J., & Huettel, S.A. (1998). Males and
females use different distal cues in a virtual environment
navigation task. Cognitive Brain Research, 6, 351-360.
Schmidt-Koenig, K., & Walcott, C. (1978). Tracks of pigeons homing
with frosted lenses. Animal Behaviour, 26, 480-486.
Sherry, D. F., & Vaccarino, A. L. (1989). Hippocampus and memory for
food caches in black-capped chickadees. Behavioral Neuroscience,
103, 308-318.
Sherry, D.F., Vaccarino, A.L., Buckenham, K., & Herz, R.S. (1989).
The hippocampal complex of food-storing birds. Brain, Behavior,
and Evolution, 34, 308-317.
Shettleworth, S.J., Krebs, J.R., Healy, S.D., & Thomas, C.M. (1990).
Spatial memory of food-storing tits (Parus ater and P.
atricapillus): Comparison of storing and nonstoring tasks.
Journal of Comparative Psychology, 104, 71-81.
Silverman, I., Choi, J., Mackewn, A., Fisher, M., Moro, J., &
Olshansky, E. (2000). Evolved mechanisms underlying wayfinding:
Further studies on the hunter-gatherer theory of spatial sex
differences. Evolution and Human Behavior, 21,
201-213.
Spencer, C., & Weetman, M. (1981). The microgenesis of cognitive
maps - A longitudinal-study of new residents of an urban area.
Transactions of the Institute of British Geographers, 6,
375-384.
Warren, S. G., & Juraska, J.M. (1997). Spatial and nonspatial
learning across the rat estrous cycle. Behavioral Neuroscience,
111, 259-266.
White, E.M., Goncalves, D.M., Partridge, J.C., & Oliveira, R.F.
(2004). Vision and visual variation in the peacock blenny.
Journal of Fish Biology, 65, 227-250.
Wilkie, D.M., Willson, R.J., & Kardal, S. (1989). Pigeons
discriminate pictures of a geographic location. Animal Learning &
Behavior, 17, 163-171.
Williams, C.L., Barnett, A. M., & Meck, W.H. (1990). Organizational
effects of gonadal secretions on sexual differentiation in spatial
memory. Behavioral Neuroscience, 104, 84-97.
Williams, C.L., & Meck, W.H. (1991). The organizational effects of
gonadal steroids on sexually dimorphic spatial ability.
Psychoneuroendocrinology, 16, 155-176.
©2006 All copyrights for the individual chapters are retained by the
authors. All other material in this book is copyrighted by the
editor, unless noted otherwise. If there has been an error with
regards to unacknowledged copyrighted material, please contact the
editor immediately so that this can be corrected. Permissions for
using material in this book should be sent to the editor.