|
Husband & Shimizu Home Page
Next Section:
Taking Flight: Post Retinal
Processing
___________
|
IV. Evolution of Retinal Structures
Despite the sophisticated visual abilities
that birds and primates share, the visual systems of non-mammalian amniotes
(reptiles and birds) contain some interesting differences compared to primates.
The answer to the question of whether the visual systems of birds are qualitatively
or quantitatively different may lie in the particulars of their retinal
structures and the organization of their central visual pathways.
The following data concentrates on that derived primarily from studies
in the pigeon (Columba livia).
Retinal Morphology and
Processing
The basic retinal structure of vertebrates
is very similar, usually characterized by the presence of five major layers
and five major cell types. The five layers are the outer nuclear
and plexiform layers, the inner nuclear and plexiform layers, and the ganglion
cell layer.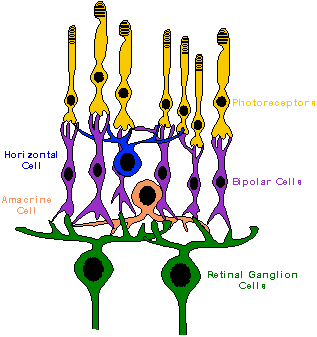 Five major classes of retinal neurons are also recognized: photoreceptors,
bipolar cells, horizontal cells, amacrine cells, and ganglion cells.
The photoreceptors (rods and cones), bipolar cells, and horizontal cells
make synaptic contacts with each other in the outer plexiform layers. The
bipolar, amacrine, and ganglion cells make contact in the inner plexiform
layers.
Five major classes of retinal neurons are also recognized: photoreceptors,
bipolar cells, horizontal cells, amacrine cells, and ganglion cells.
The photoreceptors (rods and cones), bipolar cells, and horizontal cells
make synaptic contacts with each other in the outer plexiform layers. The
bipolar, amacrine, and ganglion cells make contact in the inner plexiform
layers.
Despite basic similarities with primates,
the avian retina contains important differences in the number, ratio, distribution,
and morphological subclasses of retinal cells, and in their physiological
responses. For example, the avian inner nuclear layer is especially
rich in horizontal and amacrine cells compared to that of primates.
The anatomist S. Ramón y Cajal noted the complexity of inter-connections
in the avian retina; he thought this probably represented a great deal
of intra-retina visual processing (Rodieck, 1973). This may reflect
an important difference between primate and avian visual systems in that
complex processing which occurs in higher forebrain areas in primates may
be achieved at a lower level in birds.
Photoreceptor
& photopigment evolution. Which of the two primary classes, rods
or cones, is the ancestral photoreceptor? Given the tremendous
variation seen photoreceptors across vertebrate and invertebrate species,
this in not an easy question to answer based on simple phylogenetic assumptions.
In addition, it is often difficult to clearly distinguish certain rod and
cone types from each other, or classify them into one or the other category.
Rods appear to be relatively more conserved in vertebrates in terms of
pigments and structure than cones, and therefore could be considered the
more ancestral form. However, rods in some respects are more morphologically
complex than cones, having developed extreme sensitivity (capable of detecting
as little as one photon of light). The cyclostomes
(e.g., lampreys and hagfishes) descended from a now extinct line of jawless,
fish-like vertebrates of the Devonian period. These organisms are
thought to have a similar morphology to some of the earliest vertrbrates
(Bowmaker, 1991). Lampreys have two classes of photoreceptors, consisting
of a short outer-segment type and a long outer-segment type. It is
still debated whether these are rods or cones. Nevertheless, lampreys
appear to have duplex retinae with receptors demonstrating wavelength sensitivity
maxima at 517nm for the shorter, more "rod-like" type and 555nm for the
longer, more "cone-like" type.
Based on several
lines of evidence the ancestral vertebrate visual system probably
had a relatively unspecialized photoreceptor that was not strictly classifiable
as either a rod or cone.
This receptor probably exhibited two spectral classes of photopigment at
a very early stage in evolution (Bowmaker, 1991). The radiation of
amphibians, reptiles, birds, and mammals led to immense changes in photoreceptors
and pigment types due to the differing demands of their operating environments.
The functional spectral range of photopigments
is constrained by several factors. Vertebrates have photopigments
with ranges of maximum spectral sensitivity from the ultraviolet (350nm)
to the far red (630nm). The earth's atmosphere filters much of the
spectral radiation, and the 80% which reaches the earth's surface is in
the 300-1100nm range. The energy distribution has a maximum of 480nm
and a quantum distribution with a maximum at 555nm (Bowmaker, 1991).
Photons above 850nm are not energetic enough to photoisomerize organic
molecules, and photons below 300nm can be destructive to organic molecules
(Bowmaker, 1991). Thus, maximum biologically relevant range is about
300-850nm. This range is itself often constrained by ecological factors,
for example the photopigments of deep sea fish. Light traveling through
the water is reduced at both short and long wavelengths; at increasing
depths the light is not only attenuated but is reduced first at longer
wavelengths, and then shorter ones as the depth increases. Most deep
sea fish have all rod retinas, with maximum sensitivities at 470-480nm,
which matches what would be predicted from the wavelength transmission
properties of clear water (Bowmaker, 1991). Opponent-processing functions in color
vision probably evolved relatively early in the history of vertebrate visual
systems. Opponent processing has been found in the retina of
the bowfin fish Amia calva, a species considered one of the most unchanged
or conserved from its evolutionary ancestry (Burkhardt et al., 1983).
The presence of an opponent processing system in this "hold-over" from
the dinosaurs, and its existence in a variety of fish, reptiles, birds,
and mammals, indicates that such a system evolved very early as a mechanism
for spectral differentiation. It most likely originated in some common
vertebrate ancestor many millions of years ago rather than through convergent
evolutionary processes.
Avian
Retina
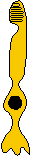 Photoreceptors
- double cones. Although the avian retina has a duplex
nature (i.e., containing both rods and cones) similar to that of primates,
it also possesses (along with reptiles, many fish, and amphibians) photoreceptors
known as double cones. In fact, all vertebrate classes, with
the notable exception of placental mammals, possess such double cones (Sillman,
1973). The double cone consists of a principle cone (similar
in structure to a normal single cone) and an accessory cone which
curves around the inner segment of the principle cone (see Figure).
In the scheme of photoreceptor evolution proposed by Walls (1942), the
double cones were probably present in the Captorhinda (ancestral
amniotes) and possibly in the dinosaurs as well. Photoreceptors
- double cones. Although the avian retina has a duplex
nature (i.e., containing both rods and cones) similar to that of primates,
it also possesses (along with reptiles, many fish, and amphibians) photoreceptors
known as double cones. In fact, all vertebrate classes, with
the notable exception of placental mammals, possess such double cones (Sillman,
1973). The double cone consists of a principle cone (similar
in structure to a normal single cone) and an accessory cone which
curves around the inner segment of the principle cone (see Figure).
In the scheme of photoreceptor evolution proposed by Walls (1942), the
double cones were probably present in the Captorhinda (ancestral
amniotes) and possibly in the dinosaurs as well.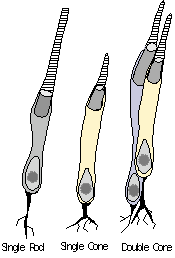 The principle and accessory segments
of the double cone may contain different visual pigments. The interaction
of signals from cones with different pigment types is crucial to color
vision. The electrical signals generated by the double cones in response
to different wavelengths of light indicate that each member of the pair
has strong interactions with the other. This may be functionally
comparable to the type of interaction which occurs in primates, but in
the context of single cones in a trichromatic system influencing the retinal
ganglion cells. Hence, whereas initial color processing occurs at
the ganglion cell level in primates, the avian visual system may perform
color processing steps within the photoreceptors themselves. Despite
the differences inherent in these systems, they appear to achieve comparable
color abilities. Avian
photoreceptors - oil droplets. The single cones, as well as one or
both segments of double cones, may also contain an oil droplet.
Oil droplets are a common feature of the cones in many vertebrate retinas,
but are rarely found in rods. These drops consist of lipids in which
carotenoid pigments are dissolved; they can appear transparent or clear,
pale yellow, green, orange, or red. They are positioned at the distal
end of the inner cone segments, covering the entire width of the receptor.
Light passes through the droplet before entering the photosensitive outer
segment. Colored oil droplets are presumed to have evolved from the
colorless cone forms found in other vertebrates like the fish coelacanth
(Bowmaker, 1991). Evidence suggests that oil droplets are more rapidly
influenced by natural selection that opsins (photopigment elements); (Varela
et al., 1993). Oil droplets were probably present in the retina of
Captorhinda and most likely in the dinosaurs.
Oil droplets, they are widely thought
to act as cut-off filters, absorbing light below their characteristic wavelengths
of transmission and conveying longer wavelengths to their associated
photopigments.
This would have a net effect of shifting maximal sensitivity towards longer
light wavelengths. Other theories of oil droplet function include
the reduction of chromatic aberration and enhancement of contrast.
For instance, Walls (1942) argued that oil droplets probably do not serve
a color function because the avian fovea is devoid of any red oil droplets.
He concluded that it was not logical for the area thought to have the highest
acuity to lack color discrimination ability at this end of the spectrum.
Yellow oil droplets could function to reduce the chromatic aberration
and scattering attributable to shorter wavelengths. This would be
similar to the reduction of short wavelength scattering performed by the
yellowed lenses in animals like diurnal squirrels and humans (Walls, 1942).
Additionally, Walls suggested the red and yellow droplets could both serve
to enhance contrast under different conditions. For example,
yellow droplets would help enhance contrast between an object as seen against
a blue sky, while red droplets would reduce the effect of a green background
(e.g., viewing an object on the ground). A high density of red and
orange oil droplets in the pigeon retina are found in the superior dorsal
quadrant, an area that has been called the "red field." Its shape
is approximately circular and occupies almost all of the superior dorsal
quadrant of the retina, extending nearly to the fovea. The remainder
of the retina in the pigeon is often termed the "yellow field." It
has been suggested the pigeon retina's ventro-nasal yellow field is in
an ideal position for viewing the blue sky, and concurrently the retina's
dorso-temporal red field is well suited for viewing the green ground (Sillman,
1973). For more details on the implications of these areas of the
pigeon retina, see the chapter by Blough, 2001
on visual search.
Colorless oil droplets found in many
birds may relate to the ability to perceive very short spectral wavelengths
(e.g., ultraviolet or near ultraviolet). The yellow tint of the
primate lens filters out such wavelengths, and cones containing photopigments
sensitive to ultraviolet (UV) or near ultraviolet wavelengths (and presumably
the ability to perceive such have not been found in primates. There
is evidence, however, that such mechanisms exist in a wide a variety of
other organisms like honeybees, fish, and birds. The performance
of visual discriminations based on UV wavelengths indicates the pigeon
has the ability to detect such light; however it has been very difficult
to chemically isolate the photopigment which performs this function.
UV wavelengths could serve a signaling function, since bird plumage
tends to have shorter wavelength reflectance than many other natural objects
(Varela et al., 1993). Ultraviolet reflectance could also be used
as a cue in discriminating foods (e.g., plants, seeds, berries)
or other natural objects. Sensitivity to UV may also play a role
in aerial navigation as an adaptation to the coloration of an unclouded
sky (Valera et al., 1993). Short-wavelength gradients would vary
depending on the sun's angle in the sky. These gradients would increase
in saturation as the sun moves from directly overhead to an angle of 90
degrees. Color gradients in the sky due to UV or polarized light
may also assist navigation when the sun is hidden by clouds. It is
difficult to appreciate the possibly rich realm of a UV "color" space,
since we have no direct experience with it. Birds appear to have excellent color
vision, which may be based on as many as four or five different photopigments. This contrasts with the three cone pigments in primates. (See
Figure 1)Evidence
for tetra- or pentachromatic vision in birds versus the trichromacy present
in primates comes from analysis of the chemical properties  of visual pigments
and behavioral discriminations. Pigeons possess a rod photopigment
(rhodopsin) with a maxima of 500nm, and iodopsin and related cone pigments
with maxima at about 413, 467, 514, and 567nm (Varela et al., 1993).
Behaviorally, the best color discrimination in pigeons occurs at 460, 530,
and 595nm (Emmerton & Delius, 1980). Pigeons can also discriminate
wavelengths toward the ultraviolet end of the spectrum, in the range of
360-380nm. of visual pigments
and behavioral discriminations. Pigeons possess a rod photopigment
(rhodopsin) with a maxima of 500nm, and iodopsin and related cone pigments
with maxima at about 413, 467, 514, and 567nm (Varela et al., 1993).
Behaviorally, the best color discrimination in pigeons occurs at 460, 530,
and 595nm (Emmerton & Delius, 1980). Pigeons can also discriminate
wavelengths toward the ultraviolet end of the spectrum, in the range of
360-380nm.
The inner nuclear layer - bipolar cells. Common
to all vertebrate retinae are bipolar cells which show center-surround
opponency and separate ON- and OFF- center types. The defining
characteristic of bipolar cells is their dendritic contact with
the photoreceptors. The bipolar cells of birds, reptiles, and amphibians
are very similar, consisting of two types recognized by the anatomist Ramon
Cajal: outer (or large) and inner (or small) bipolar cells (Rodieck,
1973). Cajal also noted that the inner plexiform layer of birds is
very thick compared to the other amniotes (Rodieck, 1973). In mammals,
bipolar cells tend to share characteristics with teleost fish rather than
reptiles or birds, and consist of three recognized types: rod, cone, and
giant bipolars (Rodieck, 1973). An important difference across species
is the degree to which the bipolar cells arborize into more than one sublamina
of the inner plexiform layer. The echidna (one of the monotremes)
shares this trait with several non-mammalian species. It has been
suggested that this organization indicates more mixing of the ON- and OFF-
pathways in non-mammalian retinas (Rodieck, 1973). This could have
effects on the processing of weak contrast targets and backgrounds, or
the ability to ascertain rapid intensity shifts across the retina.
If the degree of inter-sublaminar spread is functionally significant, it
may indicate a higher sensitivity to low spatial frequencies or motion
detection in non-mammalian amniotes compared to primates. Conversely,
such functions in primates could be compensated for by more central mechanisms,
perhaps in the tectum or higher areas. The
inner nuclear layer - horizontal cells. The
horizontal and amacrine cells serve to mediate the lateral spread of visual
activation in the retina. Horizontal cells perform this modulation
in the outer plexiform layer. Cajal distinguished two types of horizontal
cells common to birds and reptiles, brush-shaped and stellate (Rodieck,
1973). In mammals and amphibians, Cajal distinguished two somewhat
different classes, the inner and outer horizontal cells. The number
of horizontal cells is mostly consistent across fish, reptiles, birds,
and mammals. However, Cajal noted a correlation of rod structure
and density to the size of the horizontal cells (Rodieck,
1973). In mammals and teleost fishes, the rods are somewhat thin and associated
with large horizontal cells, while in birds and reptiles the rods tend
to be associated with smaller horizontal cells.
Physiologically,
horizontal cells can be divided into two general t ypes (Rodieck, 1973):
1) those that hyperpolarize or depolarize
depending on the stimulating wavelength (chromaticity or C-type)
2) those that hyperpolarize to light regardless
of wavelength (luminosity or L-type)
The C-type is usually found in species
of fish with good color vision, but interestingly, avian and mammalian
horizontal cells are of the L-type. The significance of this difference
in fish versus amniotes, who have somewhat comparable color capabilities,
is unknown. One obvious possibility may be that the C-type horizontal
cell is specialized to respond to the spectral conditions unique to the
underwater environment.
The
inner nuclear layer - amacrine cells. Amacrine
cells function similarly to horizontal cells in transferring information
laterally across the retina. There are over 20 morphological types
of amacrine cells, which use at least eight different neurotransmitters
(Kandel et al., 1991). Amacrines may also shape the complex receptive
fields seen in some retinal ganglion cells. They are in a position
to modulate antagonistic inputs from bipolar cells to the receptive field
surrounds of ganglion cells. The complexity of ganglion cell receptive
fields usually correlates with the amount of amacrine cell input.
The ON-OFF retinal ganglion cells with directional selectivity receive
input from amacrine cells (Dowling, 1990). Amacrine cells are very
diverse between amniote forms and even within members of the same species.
In addition, there are regional variations in amacrine cell density.
For example, in the pigeon there are high numbers of amacrine cells found
in the red field (dorsotemporal quadrant) of the retina (Nalbach et al.,
1993). The predominance of complex responses and directional selectivity
in avian retinal ganglion cells compared to those in mammals may be attributable
to amacrine circuitry.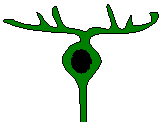 Retinal
ganglion cells. There
are wide differences in the number, types, and distribution of retinal
ganglion cells (RGCs) between birds and primates. There
are 1 million RGCs in rhesus monkey and man compared to 2 million or more
in chick, pigeon, and quail (Thompson, 1991). In general, nocturnal
animals and deep-sea fish tend to have lower quantities of ganglion
cells, and in other organisms the number of ganglion cells correlates well
with the number of cones present in their retina. The large variation
seen in RGCs make them difficult to classify. Both morphological
and physiological criteria for recognized classes have shifted as different
variations are discovered and described. Early classifications by
dendritic fields or response types (ON-, OFF-, and ON-OFF) have become
uncertain given new data, which has extended the known diversity in their
morphology and response properties. RGCs in birds possess very complex
receptive fields; along with the simple luminosity-responsive cells, there
are some which exhibit their most vigorous responses to horizontal or vertical
stimuli, and stationary or moving edges. These responses in the bird
are similar to those found in the RGCs of reptiles and amphibians (Sillman,
1973).
In general, there is an inverse correlation
between the presumed complexity of visual analysis occurring in the RGC
layer and the importance of visual cortex. It has been suggested
that the complexity of RGC receptive fields is related to the extent of
binocular processing in the animal. This relationship is one of reduced
RGC response-complexity in those organisms exhibiting larger binocular
fields or extensive binocular processing. For example, owls, with
their frontally-placed eyes and highly developed retino-thalamic connections
to visual Wulst, have ganglion cells which tend toward simpler ON- or OF-center
units (Pettigrew, 1978). These RGC properties are also found in primate
retina. The relative simplicity of RGC receptive field responses
in owls and primates versus that of more lateral-eyed birds and mammals
may related to the "correspondence problem." For stereovision to
occur at the retinal level there would have to be a large quantity of ganglion
cells to code the enormous number of possible orientation, direction, and
size parameters to match before binocular processes continue to telencephalic
levels. The general idea that "complex processing" occurs at the
level of the ganglion cells in birds but has been shifted to diencephalic
or telencephalic levels in primates may be too gross a generalization.
There are other interpretations of the data relating to binocular vision.
To quote Pettigrew (1991): "Differences in the proportion of the
specialized retinal ganglion cells turn out, then, to be quantitative rather
than qualitative, and provide the following useful generalization: the
proportion of concentrically organized, non-specialized retinal ganglion cells,
as a function of the highly specialized retinal ganglion cells, tends to
increase with the importance of binocular vision for the animal."
It may be that simple-response
ganglion cells developed as derivations after the retino-tectal system
was in place. Most of the complex RGC receptive fields that are found
in mammals are of retino-collicular cells. These cells have physiological
properties similar to those seen in nonmammalian vertebrates. In
mammals and possibly birds, visual evolution may progress with the addition
of specific retino-geniculate ganglion cell types and connections to the
existing retino-collicular groups. The distribution of RGCs differs widely
across organisms. High packing densities of RGCs are typically
correlated with high densities of their associated cones. These densities,
in turn, are assumed to equate with areas of high visual acuity for the
organism. Areas are sites on the retina in which the cones
become more densely packed at the expense of rods. Areas can either
be circular (an area centralis) or elongated (a visual streak).
Due to the typical 1:1 ratio of cones to ganglion cells, the density of
cones and associated cells in highly developed areas like those in diurnal
birds, lizards, and primates, would cause a large bulging of the retinal layer. This bulging does not occur, however, due to the development
of a pit within the area, called a fovea, which is formed by the
lateral displacement of the proximal neurons and fibers. Foveal pits
tend to be more pronounced and deeper in diurnal birds in comparison to
primates (including humans). Even in the absence of a distinct pit,
many organisms have areas of higher photoreceptor concentrations in certain
regions of the retina.
|
Foveae
in a diurnal sea bird (Arctic Tern; top) & primate (human,
bottom).
 |
The distribution of RGCs tends to correlate
better with behavior and habitat than phylogeny (Thompson, 1991).
Therefore, while RGC distribution may be unreliable in terms of reconstructing
phylogenies, there is good evidence that comparisons of RGC densities across
species can yield important information regarding the visual worlds of
these organisms.
Regional differences in ganglion cell
distributions have typically been associated with where in the visual field
important events requiring high detail vision would occur for the organism
(Thompson, 1991). In birds, there can be a single or multiple
areas, a horizontal streak, or both. Owls possess a single fovea,
but instead of being located centrally it is more temporally placed.
Birds whose feeding strategies necessitate good speed and distance estimation
in daylight (e.g., hawks eagles, and terns feeding "on the wing") have
both central and temporal foveae, with the temporal foveae presumably assisting
in good stereoscopic depth perception. Visual streaks are usually
associated with the horizontal meridian. An example of a bird with
such a visual streak is the white-capped albatross; this streak may serve
to enhance sensitivity to objects on the horizon when flying or feeding
over the surface of the water (Sillman, 1973). A more lateral-eyed
bird like a pigeon has two areas:
xxx1) a shallow
central fovea, located slightly ventral to the horizontal median and posterior
to the vertical median and
xxx2) an
area located in the posterior dorsal quadrant which is almost as rich in
cells as the fovea itself.
The central area may be used for monocular
viewing of objects in the lateral visual field, while the superior dorsal
area may assist in viewing objects binocularly in front of the birds (e.g.,
in both predatory owls or ground-feeding species like the pigeon).
Most birds (e.g., crows and sparrows) fall into the category of central
monofoveal, with a single fovea near the center, slightly above and nasal
to the optic disc. Some birds, such as the domestic chicken and California
quail, do not appear to have a fovea (Sillman, 1973).
Comparing
Avian and Primate Retinae
Is the avian retina "special?"
The avian eye possess structures not found in the eye of primates. When
studying the avian visual system, it is natural to make comparisons between
it and the visual system in humans and non-human primates. The presence
of only two basic photoreceptor types (single rods and cones) and lack
of oil droplets in placental mammals may lead to the impression that birds
have an unusally complex retinal structure compared to other organisms,
including mammals. This is not the case; the avian retina (with a few exceptions)
is very similar to that of modern reptiles. In terms of the comparative
anatomy of the retina, it is the placental mammals, and especially the
primates, who are the "odd ones" among the vertebrates.
Mammalian retina. The differences in the eye and retina
among the three mammalian classifications are instructive in determining
the overall course of visual system evolution. Given the separate
lineages and evolutionary history of monotremes, marsupials, and placentals,
one cannot consider modern monotremes or marsupials
as an early stage of the placentals. However, given the similarities
of the monotreme eye with the reptilian, it could be argued that in at
least their visual system they are sufficiently conserved over their evolution
from their reptilian forebears to make useful comparisons against the marsupials
and placental mammals.
The monotreme eye, with the exception
of the oculo-rotary muscles and the ciliary body, is very similar to that
of a reptiles and birds. So similar, in fact, that Walls (1942)
suggested that it might easily be mistaken for the eye of a nocturnal reptile.
The monotreme retina extends farther temporally than nasally, perhaps suggesting
the importance of the binocular field (Walls, 1942). The echidna
has a pure rod retina, with three outer layers of nuclei and two inner
layers. There is a single row of scattered ganglion cells.
According to Walls (1942) the photoreceptors are
probably derivatives of those in sauropsida. In the platypus the
single cones and double cones still exhibit oil-droplets, but these are
mostly colorless. The rod and cone nuclei are not well differentiated,
both being cone like. The absence of cones in the echidna and their
simplification in the platypus are likely the consequence of adaptation
to a nocturnal (or at least dim-light) lifestyle.
The marsupial eye seems to possess both
mammalian and reptilian characteristics.
Walls (1942) characterized the marsupials (e.g., opposums, kangaroos) as
having mammalian eyes (i.e., eye shape, lens, etc.) but a more reptilian
retina. This reptile-like retina is similar to that found
in monotremes. The photoreceptors are similar to those in the platypus,
with both single cones and double cones with oil droplets, and slender
rods. The single and double cones are very similar to their corresponding
types in reptiles.
The placental mammals seem to have "lost"
the double cones and oil droplets characteristic of their reptilian forebears.
Most placental mammals have duplex retinae, and generally the placental
retina is simpler than that of other amniotes. Many placental mammals appear
to have no cones (e.g., armadillo, bats, hedgehogs); those that do possess
only single cones without oil-droplets. No placental is known to
have double cones or oil droplets in its single cones. The ganglion
cells usually form one layer, except in an area centralis or in the fovea,
such as that found in primates. It would appear that the stem ancestral
placental mammal must have lost the double cones and oil droplets, undergoing
a kind of simplification, at least of the retinal structures. Walls
(1942) presumes, based on the transition from striated to unstriated intra-ocular
muscles in the reptile-monotreme transition, that accommodation became
unimportant for early mammals. This may be because it was never necessary
for them to close the pupil quickly, implying that they operated primarily
under scotopic and/or nocturnal conditions. It is commonly held that
at some point in their evolution, the mammals went through a period of
nocturnal activity, probably coinciding with the ascent of the dinosaurs.
Bottleneck
Theory of Mammalian Evolution
| "The
eye of man, with its pretty good accommodation, its fovea, its miscellaneous
yellow filters, and its capacity for color vision, possesses in substantial
degree the physiological capabilities of the standard sauropsidan
eye as we see it in the lizard or the bird. But it has gained these
powers through a lengthy process of re-differentiation, which was
carried out largely within the confines of the primate order itself."
Walls (1942); reprinted
in Cronly-Dillon & Gregory (1991), pg. 478 |
The "Bottleneck Theory" proposed by Walls
(1942) asserts that during the rule of the dinosaurs, the ancestral mammals
were forced out of a diurnal lifestyle to a nocturnal one. The
ancestral mammal probably was a small-eyed, nocturnal insectivore (and
an accompanying rod-dominated retina is assume. These mammals presumably
possessed the double cones and oil droplets inherited from their reptilian
ancestors. This includes a well-developed collothalamic visual system.
During the "Age of Reptiles", with most of the land areas inhabited by
numerous carnivorous dinosaurs. The mammals were forced by predation
pressures to adapt a more nocturnal lifestyle. During this
period, the cones underwent simplification, resulting in a predominantly
rod-containing retina. The oil droplets disappeared, or became colorless,
since scotopic conditions prevailed for these forms. The early placentals
had large pupils, simple large lenses with unstriated intra-ocular muscles
which did not support accommodation. Placentilian retina has single
cones with no oil droplets or other special features found in other amniote
cones. In essence, the placental mammals have cones reduced to their
simplest form (Walls, 1942). Only in the primates do the cones exhibit
such a sophisticated capacity for color vision. If the duplex retina
persisted throughout the placental line early on, then one would think
that all mammals, and not just the simians, would have retained a complete
color vision system like that found in birds. It is more likely that
at one time all living placentals had rod-only retinae and that subsequent
placentals evolved duplex retina from pure rod ones.
After mammals escaped the evolutionary
bottleneck, they reacquired cones and the capacity for color vision.
After the mass extinction of the dinosaurs at the Cretaceous-Tertiary boundary
(the KT boundary, about 65 MYA), mammals "passed through" the evolutionary
bottleneck. Released from the predation pressures of the dinosaurs,
they were relatively free to rapidly develop and expand into various new
ecological niches that were opened up for them. The return to diurnal
niches of some forms led to the reemergence of color vision. Color
vision appears to have evolved twice separately in the mammalian line with
the diurnal squirrels and diurnal primates.
Support for the bottleneck theory comes
from analysis of the visual system in snakes. Perhaps surprisingly,
support for this theory comes from comparing the visual systems of placental
mammals and snakes. Evidence suggests that snakes arose from diurnal
lizards which adapted a burrowing lifestyle and subsequently underwent
the atrophy and eventual loss of the limbs. Both the snakes and placental
mammals went from being nocturnal to diurnal (snakes lost their legs, went
burrowing, then came back up). The eye of placental mammals and snakes
is more spherical than birds and other reptiles. Their spherical
eye possesses a lens which acquired a yellow tint, substituting for the
yellow oil drops lost from a reptile ancestor. During their burrowing
period, the snake eye lost their "reptilian" oil droplets and double cones.
Upon re-emergence above ground, the early snakes (Boidae, including modern
pythons and boas) gained rods containing rhodopsin and single cones (Walls,
1942). In a sense, the snake visual system "devolved", and
it was reinvented, as in the placental mammals.
To summarize, the retina of reptiles
and birds might have evolved differently from the primates primarily because
of the period of nocturnal existence that early mammals underwent.
One can look to the time when dinosaurs ruled as one of the pivotal points
in the evolution of the mammalian eye and retina. During this period,
the birds maintained a predominantly diurnal lifestyle, relatively safe
from predation pressures from the dinosaurs because of their ability to
fly. From the early placental mammals, the primates eventually evolved
superior color and object recognition abilities.
Next Section: Taking Flight: Post-Retinal
Processing
|

